Abstract
Over the past two decades, invasive coronary physiology assessment has advanced significantly. Despite the proven prognostic significance provided by invasive physiological assessment of lesions by means of fractional flow reserve or adenosine-free non-hyperaemic pressure ratios, challenges in clinical practice hinder widespread adoption and limit additional value for optimising percutaneous coronary intervention decisions. Despite notable progress, uncertainties persist, emphasising the need for further research to establish a single numerical parameter in the diagnosis of a functionally significant disease, clarify the impact of longitudinal vessel analysis, and support the relevance of pressure indices in post-intervention optimisation.
Key Points
1. This manuscript aims to increase awareness of recent advancements in coronary physiology, highlighting the valuable role of integrating physiology indices into daily practice.2. The development of a single numerical parameter (one-point number), along with the default performance of longitudinal physiological vessel analysis, could improve daily efficiency of percutaneous interventions and inform future clinical outcomes. Post-percutaneous coronary intervention physiology indices are able to guide optimisation strategies; nevertheless, a certain degree of uncertainty persists, especially for optimal cut-off points.
3. Significant advances in coronary physiology have been made. Their comprehensive application in modern catheterisation labs is crucial at every stage of coronary interventions. Future insights will further refine clinical decision-making processes.
INTRODUCTION
In the past two decades, since Andreas Gruentzig’s pioneering work introducing trans-lesional pressure gradient assessment as index of percutaneous coronary intervention (PCI) success,1 invasive coronary physiology assessment has witnessed a marked improvement.2 Various indexes derived from coronary blood pressure have been developed, with randomised trials supporting their use to improve PCI-related clinical benefits.3-7 Despite the established prognostic significance outlined in the current guidelines,8,9 there are still challenges in clinical practice, either hindering its widespread adoption or limiting its additional value for deciding and optimising PCI. Addressing these uncertainties is of paramount importance for increasing their application and improving patient outcomes, bringing coronary physiology to a modern role in the catheterisation (cath) lab.
Ongoing investigations primarily focus on three pivotal aspects to bridge the historical role of coronary physiology in the modern cath lab: 1) the meaning of a singular numerical parameter (i.e., one-point number); 2) the value of longitudinal physiological vessel analysis; and 3) the role of pressure indices in post-PCI optimisation (Figure 1).
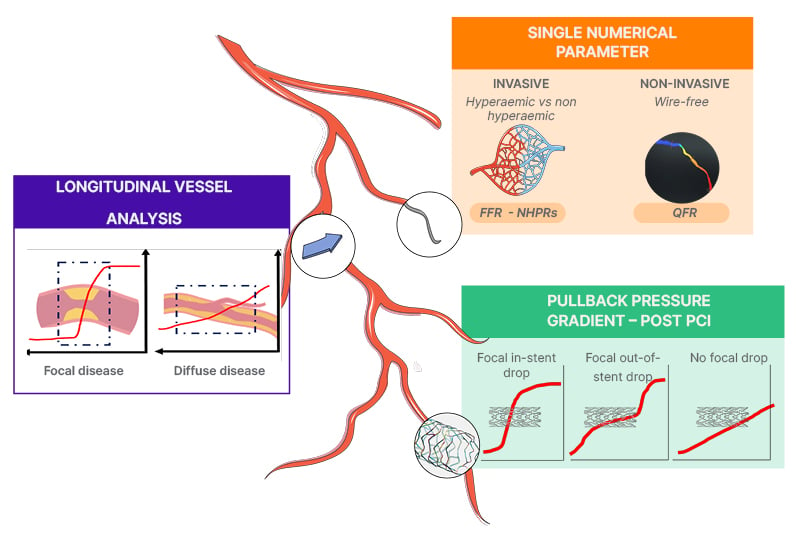
Figure 1: Coronary physiology in the modern catheterisation lab.
FFR: fractional flow reserve; NHPR: non-hyperaemic pressure ratios; PCI: percutaneous coronary intervention.
THE MEANING OF A SINGULAR NUMERICAL PARAMETER
Current guidelines advocate for invasive physiological assessment using fractional flow reserve (FFR) to identify ischaemia-causing coronary stenosis in patients with intermediate lesions or uncertain evidence for lesion-based ischaemia, guiding revascularisation decisions.8,9 Adenosine-free non-hyperaemic pressure ratios (NHPR) have emerged as a simpler alternative to FFR, supported by two large randomised trials demonstrating no significant difference in 5-year hard clinical endpoints between instantaneous-free ratio (iFR)-guided and FFR-guided PCI strategies.6,7 Notably, as an answer to address the perceived underuse of wire-based physiological assessments, attributed to longer procedural time, need for adequate training, potential complications from pressure wire instrumentation, and costs,10 consistent advances have been made with the development of wire-free image-based approaches.11 Among the available computational solutions providing acceptable diagnostic accuracy (quantitative flow ratio [QFR], coronary angiography-derived fractional flow reserve, vessel fractional flow reserve, and Murray law-based quantitative flow ratio), QFR, derived from three-dimensional coronary reconstruction and fluid dynamics computations from the angiogram, stands out as the sole angiography-based physiological index with prospective validation; demonstrating a substantial lesion reclassification (~20%) and improved 1-year and 2-year clinical outcomes compared to conventional angiography in different subsets.12-16 However, limitations such as manual vessel contouring, proprietary software, nitrate dependency, single-vessel analysis, inapplicability to ostial lesions or major bifurcations, and reliance on optimal projections should be acknowledged. Interestingly, an ancillary analysis from the FAVOR-III China, although not depicting any statistical significance among pre-specified subgroups, showed a trend toward increased beneficial effect when the evaluation was performed in experienced centres.12
In this field, further improvements are expected, such as the broad application and clinical validation of intracoronary imaging-based protocols (optical flow ratio [OFR] and ultrasonic flow ration [UFR]), or the use of artificial intelligence, improving the quality of the measurements and reducing intra- and inter-observer limitations.11,17
Notably, approximately 20% of cases exhibit discordant results between FFR and NHPRs, attributed to variations in measuring the non-true resting state or to the insufficient hyperaemia, thus limiting the reliability on their results.11 Observational studies of iFR/FFR discordance suggest impaired clinical outcomes only when both parameters are abnormal, while lesions with discordant results have similar outcomes to lesions with concordant negative results, emphasising that the independent choice of one test may offer diagnostic adequacy for clinical decisions, but at the cost of eventually different approaches (i.e., PCI) based on the choice of the test.18-20 Nevertheless, the overall clinical and prognostic implications of these lesions remain unclear and open to further daily uncertainty.
On this background, future investigations should focus on anticipating clinical outcomes linked to between-tests discordance and develop a singular numerical parameter, such as a one-point number, to mitigate the intra-technique variability evident in randomised clinical trials; the inter-technique dissimilarities that contribute to overall perplexity in daily clinical practice; along with the aim to further reduce manual interactions and improve overall efficiency and global reproducibility.
LONGITUDINAL PHYSIOLOGICAL VESSEL ANALYSIS
The evolution of invasive pressure-derived indexes assessment has progressed from recognising a single value reflecting the haemodynamic impact of an index lesion, to the comprehensive analysis of the entire vessel physiology.21 In practice, the assessment of pressure variations along the entire vessel length can offer valuable insights into final PCI results and subsequent outcomes.22-25 Nevertheless, in presence of sequential lesions (i.e., tandem stenoses), accurate estimation of physiological indexes may be compromised by the “crosstalk phenomenon” among lesions (i.e., caused by the relative haemodynamic interdependence of stenoses), potentially leading to suboptimal procedural planning.2,11 In this setting, the longitudinal physiological vessel analysis may help to characterise the disease pattern (focal, tandem, diffuse) through the distribution of pressure losses along the epicardial vessel.22 Traditionally, this analysis can be performed subjectively by visual inspection of the pullback tracing, or through the pressure pullback pressure gradient (PPG) index,22 and dFFR(t)/dt index.26 The ability to discriminate between different patterns of coronary atherosclerosis carries immediate and relevant clinical implications: a focal pattern is often associated with an optimal post-PCI physiological result, since PCI results effective in removing focal flow-limiting stenosis. Conversely, a diffuse pattern of disease is frequently associated with suboptimal post-PCI results, and requires considerations on further medical therapy optimisation or surgical intervention rather than PCI.2 Notably, a sub-analysis of the TARGET-FFR trial showed that residual angina after PCI was almost twice as likely in patients with a diffuse disease, identified by a low PPG, than in patients with a focal disease (i.e., high PPG of ~1), who reported greater improvement in angina and quality of life.26-28
However, current definitions of diffuse disease are largely qualitative, and rely solely on clinical consensus statements, thus requiring further validation to provide formal and universal definitions.2,11 Importantly, most studies supporting definitions of focal, tandem and diffuse disease patterns are retrospective and relied on different diagnostic techniques, thus resulting in multiple cut-off definitions. Moreover, it has been ascertained that coronary physiology indexes, especially FFR, have a complementary role on defining plaque morphological characteristics with precise lesion features (i.e., vulnerable plaque), given the peculiar environmental composition (i.e., inflammatory mediators, etc.) and the impact on the macrovascular and microvascular circulations.29
On this background, while identifying the lesion pattern is becoming central, consensus on thresholds and techniques able to predict future outcomes remains controversial. In addition, the identification of the benefit from an integrative assessment of coronary anatomy, plaque characteristics, and physiological aspects need more research to better predict events and improve the treatment strategies.
THE ROLE OF PRESSURE INDICES IN POST-PCI OPTIMISATION
Despite the established clinical significance of pre-procedural pressure-derived indexes in assessing the functional severity of coronary stenosis, contemporary reports reveal that 20–30% of cases yield suboptimal post-PCI results, reflected by FFR values <0.8 and iFR values <0.9.30-32 Although post-PCI FFR values have been associated with future target vessel failure, cardiac death, and myocardial infarction, it is something not usually performed; moreover, there is still controversy over the optimal cut-off for defining an optimal PCI result.31,33 In case of NHPRs, a post-PCI iFR >0.95 has been related to improved outcomes in the DEFINE PCI study.31 However, universal agreement on this cut-off remains elusive. The lack of consensus stems from the fact that, although post-PCI pressure-guided optimisation can be adopted through repeated balloon inflation or stent employment, FFR-guided optimisation strategies do not often increase the proportion of patients with a final optimal FFR result (>0.90).30 Various factors may contribute to suboptimal post-PCI outcomes, including stent malapposition, plaque protrusion, thrombus within the stent, inadequate lesion coverage, incorrect stent sizing, and edge dissection.2 Nevertheless, suboptimal physiologic results after PCI may often be an epiphenomenon of diffuse atherosclerosis (bystander diffuse disease), challenging adequate revascularisation by means of PCI.2 The FFR SEARCH registry34 and reports by Piroth and colleagues35 emphasise that stent under-expansion, although not associated with a significant FFR drop, could indicate future clinical events across the entire coronary artery tree, including non-target vessels, as a result of a bystander diffuse disease. Importantly, the role of post-PCI physiology evaluation can play a crucial role in improving final results, since precise findings (i.e., location of pressure index loss) provide valid indications on the role of further stent optimisation and anomaly correction in case of in-stent or out-of-stent (i.e., proximal or distal) pressure losses, or can suggest the acceptance of the result, thus embracing alternative non-PCI approaches according to the clinical situation (i.e., no focal pressure drop).2
In this context, post-PCI assessment undeniably demands significant attention as it may provide an index for future events. However, the debate over the optimal consideration of pressure-based indexes persists, necessitating further research in this field.
CONCLUSIONS
Significant advances have been made in the field of coronary physiology; nevertheless, notable uncertainties persist. Subsequent investigations, emphasising the establishment of singular numerical parameters (i.e., one-point number), elucidating the consequence of longitudinal physiological vessel analysis in procedural planning, and affirming the pertinence of pressure indices in post-PCI optimisation, are imperative. This ongoing research aims to resolve current discrepancies, validate quantitative definitions, and challenge controversies related to optimal cut-off points, ultimately driving forward the field of coronary physiology in the modern cath lab.