Abstract
Demand for therapeutic plasma exchange (TPE) is rising as further new indications for the procedure are added to clinical guidelines. TPE systems can be categorised by the method used to separate the plasma from the cellular components of blood: membrane TPE (mTPE), in which apheresis is based on molecular size, and centrifugal TPE (cTPE), in which apheresis is based on molecular density. Until recently, both types of system were assumed to have comparable efficacy and safety, and treatment decisions have typically been influenced by local factors rather than clinical evidence. Head-to-head comparative studies over the last decade have indicated several important differences between cTPE and mTPE systems, such as greater plasma removal efficiency, shorter procedural times, more flexible vascular access options, and fewer and less severe adverse events. An anticoagulant (usually heparin or citrate) is used in both procedures; however, whereas circuit failure is common during mTPE because of blood clotting and development of a secondary membrane on the filter, to date no study has reported circuit failure attributable to clotting during cTPE. In addition to reviewing the published data from comparative and noncomparative trials in a narrative way, this article describes real-world experience and practical considerations from the nurse’s viewpoint of switching from mTPE devices to a cTPE system.
INTRODUCTION
Plasmapheresis is an extracorporeal procedure in which plasma is separated from the cellular components of the blood and then either donated or discarded and exchanged with replacement fluids. The latter approach is called therapeutic plasma exchange (TPE) first performed by the Russian physicians Vadim A. Yurevich and Nikolay Konstantinovich Rosenberg in 1913.1 In 1914, Abel et al.2 were the first to suggest the term ‘plasmapheresis’ for the treatment that has since become a well-established procedure in a broad range of conditions (Table 1).3 Most of these conditions are characterised by elevated levels of plasma components that play a pathophysiological role in many diseases, such as antibodies, cytokines, immune complexes, abnormal plasma proteins, plasma-bound toxins, and cholesterol-rich lipoproteins.3 By removing plasma components of high molecular weight, TPE can frequently interfere with key pathophysiological processes, thereby curing diseases or preventing further organ damage.4,5 One essential feature of TPE that cannot be substituted for by adsorptive procedures is the fact that it can also be used to replace large quantities of factors that are either diminished or absent in plasma, e.g., the von Willebrand factor cleaving protein ADAMTS13 in patients with thrombotic thrombocytopenic purpura.6
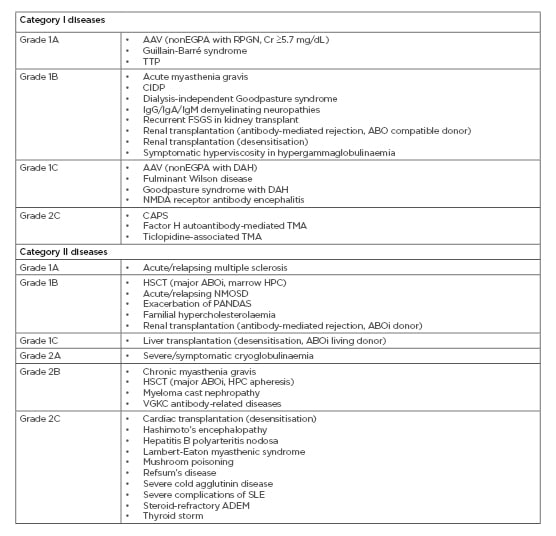
Table 1: Diseases for which therapeutic plasma exchange is accepted as first or second-line therapy (category I/II), with recommendation grades from the American Society for Apheresis (ASFA) 2019 guidelines.2
AAV: antineutrophil cytoplasmic antibody-associated vasculitis; ABOi: ABO incompatible; ADEM: acute disseminated encephalomyelitis; CAPS: catastrophic antiphospholipid syndrome; CIDP: chronic inflammatory demyelinating polyradiculoneuropathy; Cr: creatinine; DAH: diffuse alveolar haemorrhage; EGPA: eosinophilic granulomatosis with polyangiitis; FSGS: focal segmental glomerulosclerosis; HSCT: haematopoietic stem cell transplantation; NMDA: N-methyl-D-aspartate; NMOSD: neuromyelitis optica spectrum disorders; PANDAS: paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections; RPGN: rapidly progressive glomerulonephritis; SLE: systemic lupus erythematosus; TMA: thrombotic microangiopathy; TTP: thrombotic thrombocytopenic purpura; VGKC: voltage-gated potassium channel.
As the range of therapeutic indications for TPE continues to expand, demand for the procedure is also increasing.5,7 In Germany alone, nearly 27,000 TPE procedures were performed in hospitals in 2017.7 The increase in demand is particularly notable for transplant-related and neurological diseases,8,9 with treatment of Alzheimer’s disease being one of the latest investigated.10 TPE is also being used in settings for which there is a convincing pathophysiological rationale but lack of high-grade evidence (e.g., in sepsis with multi-organ failure),11,12 contributing to the rise in treatment numbers.
TPE is typically performed using systems that can be categorised into two distinct types: membrane TPE (mTPE), in which apheresis is based on molecular size, and centrifugal TPE (cTPE), in which apheresis is based on molecular density.13 During mTPE, blood plasma is separated from the cellular components using a filter that prevents the passage of cellular components and enables whole plasma removal. An anticoagulant, usually heparin, is added to the blood before it is pumped through the filter.9,13 During cTPE, centrifugation separates incoming whole blood into plasma, red blood cell, and white blood cell components. An anticoagulant, usually citrate, is added prior to centrifugation. In both procedures, the remaining cell-rich blood is mixed with a replacement fluid (e.g., albumin or fresh frozen plasma) and returned to the patient to prevent hypovolaemia.9,13
Until recently, preference for TPE method was often based on familiarity with the central concept, i.e., membrane or centrifuge rather than on data. For example, a nephrologist might be more familiar with membrane filtration than with centrifugation, and vice versa for haematologists. Although key technical differences between the two procedures have been reported over several years,15-18 in the majority of clinical scenarios, it had been assumed that mTPE and cTPE have similar efficacy overall. It was not until this assumption was tested in head-to-head (H2H) studies that the differences between the two procedures were more clearly delineated.19-22 To date, the majority of H2H studies and case reports have compared various mTPE devices to the Spectra Optia® Apheresis System (Terumo BCT, Lakewood, Colorado, USA) cTPE system. Therefore, unless otherwise stated, attributes and outcomes for cTPE reported in this article refer specifically to the Spectra Optia device and may not be generalisable to all other cTPE systems. In addition to reviewing the published H2H trials, this article includes two case studies describing, from the nurse’s viewpoint, the practical aspects of switching from mTPE devices to the Spectra Optia system.
REASONS FOR SWITCHING TO CENTRIFUGAL THERAPEUTIC PLASMA EXCHANGE FROM MEMBRANE THERAPEUTIC PLASMA EXCHANGE
Greater Plasma Removal Efficiency
Plasma removal efficiency (PRE) is a measure of the fraction of plasma removed during TPE in relation to the amount of plasma processed. PRE also correlates with the amount of anticoagulant that the patient receives during TPE; inefficient systems may lead to longer procedures, increasing the risk of anticoagulant-related toxicity.18
In mTPE, PRE is limited by plasma filtration rates and factors intrinsic to the membrane, including pore size and distribution, and is in the range of 27–53%.9,13 To avoid clotting within the filter, the filtration fraction is often limited to 30–35% of the plasma. As a result, the desired plasma clearance can only be achieved by processing 3–4 times the calculated blood volume.13 In theory, PRE is independent of blood volume processed; however, short procedures may result in lower PRE than longer procedures, taking into account the start of TPE when blood is being processed to replace the priming fluid, but no plasma is removed.9 cTPE with the Spectra Optia has been shown to have a PRE in the range of 80–93% in H2H studies versus mTPE systems and in noncomparative studies.18,20-25 PRE values reported for the COBE® Spectra Apheresis System (Terumo BCT), an earlier cTPE device and predecessor to the Spectra Optia, range from 70% to 83%.18,23-25
Shorter Total Therapeutic Plasma Exchange Time
In centres with a high volume of patients being treated with TPE, the time needed to set up and prime the systems and perform the procedure is an important consideration. Similarly, for centres seeing an increased demand for TPE, longer procedure times can amplify the pressures of space limitations and stretched resources. Every minute of nursing time saved can be put to good use. Several factors can influence total TPE time, including set up and priming, blood flow rates, and treatment interruptions (e.g., because of clotting), and wide variations have been reported for different cTPE and mTPE systems.18,22-28 Between-study comparisons are therefore unreliable.
Many mTPE devices work with balancing systems that require careful calibration when setting up each procedure. In general, cTPE systems do not require balancing and therefore need less time to set up and prime between each patient.9 In a H2H crossover study involving 27 patients, the Spectra Optia required 11 minutes on average for set up and priming, compared with 23 minutes for the Diapact® mTPE system (B Braun Avitum AG, Melsungen, Germany).21 A longer set up and priming time of approximately 40 minutes was reported in a case series describing nine procedures performed with the Prisma® mTPE system (Baxter International, Deerfield, Illinois, USA) using a TPE 2000 set.19
Because of the large differences in PRE between the two procedures, the time taken to remove the same volume of plasma may be substantially longer for mTPE than for cTPE. Across three H2H studies, standardised time to exchange 1 L of plasma (adjusted for variations in the total plasma volume of the patient) was 25–33 minutes with the Spectra Optia and 36–37 minutes with mTPE systems.9 Reported average procedure times were 91–120 minutes and 133–144 minutes, respectively.19-21 A retrospective chart review of 912 TPE procedures (185 patients) performed at a single centre in Hannover, Germany, found that cTPE procedure times were on average 15 minutes shorter than mTPE procedure times (120 versus 135 minutes; p=0.007).29
More Flexible Vascular Access Options
Importantly, blood flow rates in the H2H studies were consistently lower with cTPE despite the shorter procedure times (54–81 mL/min versus 82–150 mL/min with mTPE).19-21 In mTPE, flow rate is often increased to allow for greater PRE within an acceptable filtration fraction range, to maintain sufficient blood pressure across the filter membrane, and to reduce the risk of circuit failure attributable to blood clotting and protein clumping.15,19,21,30,31
Often, this requires placement of a central venous catheter (CVC) or creation of arteriovenous fistulae (AVF) as other access points may not provide adequate blood flow for successful apheresis (generally >40 mL/min).15,16 Interestingly, in one H2H study, in which flow rates during cTPE were kept in the low range normally used with peripheral vein access, procedure time was still shorter than that of mTPE with OctoNova® (DiaMed, Cologne, Germany) by a median of 13 minutes.20 For high-volume centres, even this relatively small difference can lead to substantial savings in nursing time. Having the additional option to perform TPE using a peripheral vein is important as poor vascular access is not uncommon in clinical practice, especially among patients requiring TPE long term.32 Moreover, peripheral vein access reduces the risk of bacteraemia compared with CVC placement.16,18
Fewer Clotting Events
Although blood clotting and protein clumping can theoretically occur in a cTPE system, e.g., in a patient with cryoglobulinaemia, so far this has not been reported.9 By contrast, H2H studies and clinical experience report a high incidence of clotting and clumping events during mTPE, usually because of the limited pore diameter in the filter.9 In up to 23% of mTPE procedures, circuit failure because of clotting or clumping means pausing the procedure to replace the filter or use another disposable set, or terminating the procedure completely.20,21,33-35
In some centres, the filter is pre-emptively changed if transmembrane pressure rises above a predefined threshold level.20 Circuit failure frequently leads to the loss of cellular and corpuscular blood components, potentially causing additional complications (e.g., anaemia attributable to blood loss).9 In one H2H study the clotting and clumping rate was as high as 67% during mTPE with a Prisma system, though this could be attributed to the different heparin
doses used.19
Fewer Side Effects
Depending on the definition, adverse events (AE) are generally infrequent during TPE.36-40 A registry analysis of >50,000 procedures in >7,000 patients reported incidences of 2.4%, 3.0%, and 0.4% for mild, moderate, and severe AE, respectively. mTPE was associated with significantly more frequent AE overall and when stratified by disease severity, compared with cTPE.40 Higher AE rates have been reported in an intensive care setting (23.9% with cTPE versus 31.7% with mTPE), but the difference was not statistically significant.33
AE of special interest include anticoagulation-induced AE and platelet loss (particularly in patients with low platelet counts related to their disease). Heparin is well known to potentially increase risk of bleeding and thrombocytopenia, a rare but serious AE occurring in up to 5% of mTPE procedures.41,42 Citrate anticoagulation is associated with increased risk of hypocalcaemia, but this tends to be mild and can be prevented with prophylactic calcium supplementation.16,43 In three H2H studies, only one case of possible mild hypocalcaemia was reported with cTPE.19-21 It is important to note that the use of citrate is not exclusive to cTPE.33,44 During mTPE, regional anticoagulation with citrate is often used in patients at high risk of bleeding, for whom heparin is contraindicated, despite a lack of strong data to support this labour intensive procedure.45 Moreover, citrate is often present in replacement fluid during mTPE.9
Platelets are at risk of being removed along with plasma during cTPE because of their relatively low specific gravity, potentially increasing the need for transfusion.46 However, despite early reports of more pronounced platelet loss during cTPE than during mTPE,47,48 current evidence suggests similar or reduced rates of platelet loss with cTPE (around 10%).20,21
Patient Satisfaction
Patient satisfaction has rarely been reported in studies using cTPE devices.49 In the authors’ experience, the shorter procedural times for cTPE versus mTPE are a key factor in patient preference. A further advantage of the Spectra Optia system and of some mTPE devices is that they offer a single-needle alternative to the usual requirement of two blood access ports for cTPE and mTPE.49,50 The single-needle approach is based on intermittent rather than continuous blood flow, which prolongs procedure time but within acceptable limits for patients. A small survey (n=5) reported 100% patient satisfaction with single-needle cTPE.49 From the clinician’s perspective, the flexibility of being able to start TPE with two lines and still be able to finish the procedure if one line fails is advantageous.
BARRIERS TO SWITCHING
Although cTPE systems offer several benefits, mTPE devices have the appeal of being multifunctional. A department with a small TPE caseload per year may not feel that they can justify purchasing a dedicated system for cTPE when mTPE can be adequately, if not optimally, performed using the same device as for haemodialysis and continuous renal replacement therapy (CRRT) procedures. It is worth noting, however, that cTPE systems can perform other therapeutic apheresis procedures that are not possible with mTPE devices, such as white blood cell depletion or peripheral blood stem cell collections. One solution might be to share a cTPE system between a blood bank and a clinical specialty such as nephrology, to enable full use of the centrifugal device.
Perhaps the most difficult barrier to overcome is to change routine behaviour, particularly in departments with a long history of performing mTPE. Describing the benefits of cTPE in terms of patient-centred care and reducing the burden on their time may help staff to see the value in switching, despite the steep learning curve they may face at the start. The question then is not which patients should receive cTPE but which patients should not.
CASE STUDY 1: WESSEX KIDNEY CENTRE, PORTSMOUTH, UK
Following an internal review, staff at a renal care unit in the south of England identified potential benefits of switching from mTPE to cTPE. Current practice at the time was to perform mTPE using a Prisma system (Prismaflex) that had been in use at the centre since 2006. An average of 25 inpatient and outpatient TPE procedures were performed each month. The Prismaflex device was also used for CRRT as well as continuous venovenous haemofiltration and slow continuous ultrafiltration. For all CRRT and mTPE procedures, anticoagulation was with unfractionated heparin. Although the device was suitable for TPE, set up time was approximately 30 minutes, circuit clogging or clotting occurred in approximately 20% of procedures and large volumes of heparin were often required, which increased the bleeding risk of the patient.
Based on their research, the nursing staff contacted Terumo BCT to arrange a trial use of the Spectra Optia for 16 consecutive TPE procedures in a 30-year-old female patient with myasthenia gravis. Prior to the trial, the patient was receiving six mTPE procedures per month. She often felt cold during the procedure and nauseous after, and prolonged treatment had resulted in the formation of a radiocephalic AVF. Circuit clogging or clotting occurred approximately once a month. Switching the patient to the Spectra Optia reduced total TPE time by around 30 minutes. Centrifugal technology had not been used at the centre before, but the nursing staff found it simple to understand and the Spectra Optia machine easy to programme. No issues were identified with clotting or clogging over the trial period, and the patient did not require a blood warmer. Postprocedural calcium levels were comparable for mTPE and cTPE when infusing calcium 2.25 mmol/L at a rate of 4.5 mmol/hour, with an average drop of 4.7% and 5.3%, respectively.
From the patient’s perspective, cTPE with the Spectra Optia resulted in less nausea following the procedure and reduced her myasthenia gravis symptoms (e.g., lethargy and blurred vision) between procedures, compared with mTPE. The patient did not experience the same ‘feeling of coldness’ during cTPE as she did during mTPE. There were no access issues attributable to the lower pump speed of the Spectra Optia system compared with Prismaflex, and no circuit clogging or clotting was observed over 16 treatments.
The main concern among nursing staff regarding switching completely to cTPE was a lack of experience with the use of regional citrate for anticoagulation. A new standard operating procedure for citrate use and calcium infusion was adopted, and by the third cTPE procedure nursing staff were able to complete the procedure entirely independently.
CASE STUDY 2: MONASH MEDICAL CENTRE, MELBOURNE, AUSTRALIA
In this case, a dialysis unit used mTPE and cTPE concurrently for 3 years before switching completely over to cTPE with the Spectra Optia. The introduction of cTPE was based on a need to improve efficiency and perform TPE more quickly, as well as a desire to have a portable device to allow the machine to travel to patients who could not attend the unit in person. Initially, there was a reluctance within the system to buy the cTPE equipment when the current mTPE equipment (Fresenius 4008; Fresenius Medical Care, Bad Homburg, Germany) was still fit for purpose. However, procedure time is crucial in a unit with limited space and high demand for TPE, and cTPE has consistently been reported as being less time-intensive than mTPE.20,21,29 The changeover to cTPE was a relatively smooth process overall. Although educational resources and support were readily available to train staff, freeing up enough time proved difficult and the centre ended up identifying ‘champions’ who were trained as super users and could pass on their learning to others. Users were given the opportunity to suggest topics for an annual symposium on TPE, and ongoing refresher courses were offered alongside training for any new staff.
At Monash Medical Centre, circuit clotting occurred in roughly 30% of mTPE procedures resulting in increased resource use (disposable sets, larger volumes of heparin, and greater demand on nursing time). Since the introduction of cTPE at the centre, no instances of clotting have been observed. A centre-based cost analysis found that cTPE with citrate was statistically significantly more cost-effective than mTPE with heparin, resulting in substantial savings and shorter treatment times.51 The average time taken to perform the cTPE procedure was 110 minutes compared to 240 minutes for mTPE. Practical benefits of the Spectra Optia over mTPE devices included the ability to reduce whole blood flow rates in patients with poor vascular access, ability to pause treatment if needed, ease of cleaning, no reliance on water, and the compact device allowing it to be moved and stored easily. Efficient use of space was critical at the centre, which until recently had only 10 chairs available for all patients referred to the service for dialysis or TPE.
An understanding of the benefits of cTPE over mTPE helped staff at the centre to appreciate the rationale for switching and nurses were reassured of the safety of citrate with respect to volume used, risk of bleeding, and hypocalcaemia. No instances of fluid overload have been observed using the standard 4% citrate anticoagulation protocol for cTPE. Similarly, no increased risk of bleeding has been observed with cTPE procedures pre- or post-surgery, in line with the Hannover experience with the Spectra Optia.20 Hafer et al.20 also reported no differences in ionised calcium levels comparing the start and the end of cTPE. Within the Monash Medical Centre system, unit-based strategies are in place for monitoring and managing serum calcium levels. At the Melbourne unit, hypocalcaemia is routinely treated with intravenous calcium gluconate 2.2 mmol/L per 10 mL; in other units, oral calcium is used with the same effect.
Based on the positive experience gained over 3 years and the preference for cTPE showed by nurses and patients, a full switch from mTPE to cTPE was made in September 2015. Ongoing training and technical support for cTPE is key to the realisation of positive patient outcomes.
CONCLUSION
The concept of TPE has been around for more than a century but it seems that the machines to perform this procedure are finally coming of age. A recent publication by Lozano et al.52 estimated that >100,000 cTPE procedures and 50,000 mTPE procedures were performed in the European Union (EU) in 2014, and treatment numbers continue to rise. In the past, TPE treatment decisions have been strongly influenced by routine and less so by evidence-based medicine and the best choice for the patient. These days, there are data from H2H clinical studies and case series to aid the choice of treatment. These studies clearly illustrate that the Spectra Optia cTPE system has several advantages over mTPE systems: greater PRE, shorter total TPE time, more flexible access options, fewer clotting events, and fewer and less severe AE. Real-world experience shows that switching from mTPE to cTPE is both feasible and advantageous, even in centres that never thought they could work without the membrane technology. Ideally, prospective trials should be carried out to investigate whether the difference in treatment time and circuit failure rate between mTPE and cTPE systems can be economically and medically justified, including centres that use multifunctional devices to perform a small number of mTPE procedures per year.








