Abstract
Cardiovascular disease is a leading cause of morbidity and mortality in end-stage renal disease (ESRD). Hypertension plays a major contributory role, resulting in progressive left ventricular hypertrophy, and increasing the risk of sudden cardiac death. The prevalence and pathophysiological mechanisms differ fundamentally from the non-dialysis-dependent population.
Sodium restriction can be as effective as antihypertensive medication in mitigating the haemodynamic effects resulting from impaired sodium handling. Tailoring dialysate sodium may enhance diffusion and facilitate greater sodium elimination where dietary measures alone prove ineffective.
Unlike hypertension in the wider population, volume overload plays a major pathophysiological role in ESRD. Probing dry weight in patients on dialysis who are seemingly euvolaemic enables clinically significant blood pressure (BP) reduction, and translates to improvements in markers of future cardiovascular morbidity and mortality.
Pharmacotherapy remains an important aspect in controlling hypertension in dialysis. Although no large-scale studies have identified the optimal medical therapy, numerous meta-analyses and randomised control trials (RCT) have demonstrated the efficacy of angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARB), calcium channel blockers, β-blockers, and hydralazine/isosorbide dinitrate in the treatment of hypertension in ESRD. Whether the beneficial haemodynamic properties of mineralocorticoid receptor antagonists outweigh the risk of hyperkalaemia is the subject of ongoing RCTs. Numerous meta-analyses have demonstrated that adequate pharmacological control of BP translates to improved cardiovascular morbidity and mortality.
The fluctuation of volume status in the inter/intra-dialytic period complicates the diagnosis of hypertension in ESRD. As with patients not receiving dialysis, 24-hour blood pressure monitoring appears to have the greatest sensitivity in diagnosing hypertension and predicting outcomes from hypertension. Where resources are limited, home BP monitoring appears to have the greatest value.
Key Points
1. Hypertension is a major contributor to morbidity and mortality in end-stage renal disease. The pathophysiological mechanisms driving hypertension are distinct compared to the general population, with fluid overload playing a major role. Other putative mechanisms include vascular stiffness, enhanced sympathetic drive, and aberrant renin-angiotensin-aldosterone system activity.
2. Management is multifactorial, with several strategies unique to dialysis patients. Modulation of dialysate sodium can enhance sodium extraction and improve volume control. Dry weight probing appears to play a leading role in treating hypertension, even in clinically euvolaemic dialysis patients. As with non-dialysis patients, fluid and sodium restriction are essential components in treating hypertension in ESRD.
3. Pharmacotherapy plays a major role in hypertension in advanced kidney disease. Pharmacological management of hypertension is uniquely complex in the dialysis cohort, owing to the greater susceptibility to side effects such as hyperkalaemia, and the influence of medication dialysability. Therefore, management of hypertension in end-stage renal failure must take into account the side effect profile, dialysability, drug efficacy, and intradialytic haemodynamics.
Hypertension is a common complication in advanced chronic kidney disease (CKD), occurring in approximately 80–85% of patients.1 Blood pressure (BP) control is suboptimal in the majority of these patients. The link between uncontrolled hypertension and mortality among patients with end-stage kidney disease (ESKD) is well documented.2,3 Much of the research on hypertension has focused on the non-dialysis population, but there are essential differences in the pathogenesis, diagnosis, and management of hypertension in patients receiving dialysis. Unlike the general population, an excess of salt and water play a dominant role in the development of hypertension in the vast majority of patients with ESKD, leading to left ventricular hypertrophy and sudden cardiac death. Numerous randomised control trials (RCT) have shown that salt restriction has a comparable efficacy to the addition of antihypertensive medication in BP control.4 Consequently, the control of salt and volume overload in these patients is the cornerstone of hypertension management. Renin-angiotensin-aldosterone system (RAAS) activation, sympathetic activation, increased arterial tone, and endothelial dysfunction are also important pathogenic factors for hypertension in patients receiving dialysis. As with patients who are dialysis-independent, antihypertensive pharmacotherapy remains a major strategy in controlling hypertension in ESKD,5 but differences in the pharmacokinetics and tolerability of certain drugs need to be taken into account. This review aims to highlight the evidence behind the diagnosis and management of hypertension in the dialysis population.
SODIUM RESTRICTION
The morbidity and mortality benefits attributed to dietary sodium restriction have been well established in cardiovascular disease and CKD.6 Due to an impaired ability to excrete a sodium load, patients receiving dialysis are considered sodium-sensitive, and yet most patients receiving dialysis consume in excess of the recommended 2 g of sodium per day.7-8 Increased dietary sodium contributes to hypertension via volume-dependent and independent mechanisms. Raised plasma sodium stimulates movement of water from intra- to extracellular compartments. Hypertonicity also stimulates thirst, again increasing the extracellular fluid compartment and intradialytic weight gain (IDWG). Independent of volume homeostasis, sodium increases medullary vasomotor sympathetic activity due to elevated angiotensin II activity.9
Dietary sodium restriction is a particularly important facet of hypertension management in patients with advanced CKD. One RCT reported that sodium restriction was associated with a 7% reduction in systolic BP.4 The beneficial antihypertensive effects of sodium restriction also translate to patients receiving dialysis.10 A recent meta-analysis looked at sodium restriction in 71 patients receiving HD, and 20 patients receiving peritoneal dialysis. The mean difference in systolic BP between low and high salt intake groups was -8.4 mmHg and -4.4 mmHg for diastolic BP. Conversely, one of the included RCTs demonstrated that the addition of 3.5 g of salt was sufficient to raise BP by 9/5 mmHg. Restricting dietary salt to <6 g/day can limit inter-dialytic weight gain to 0.8 kg by influencing thirst and extracellular volume.11 Furthermore, the HEMO study demonstrated that sodium restriction was sufficient to reduce the requirements for ultrafiltration during thrice-weekly dialysis.12 This highlights the therapeutic role of sodium restriction in managing volume overload, a major contributor to hypertension in ESKD.
DIALYSIS PRESCRIPTION
Research into the augmentation of dialysate sodium to influence inter-dialytic haemodynamics has been a subject of much controversy.13 Numerous observational studies have linked low dialysate sodium with a reduction in thirst, IDWG, and hypertension, which can improve left ventricular mass index (LVMI), a marker of cardiovascular mortality in patients on dialysis.14,15 One such observational study including 52 patients receiving dialysis demonstrated that a 3 mmol/L reduction in dialysate sodium was significantly associated with a modest reduction in BP of 5 mmHg, and even 10 mmHg in patients who are hypertensive.14 This was also associated with a reduction in pre-dialysis serum sodium, but IDWG was unaffected.
Conversely, the DOPPS trial, a large-scale international prospective cohort study which analysed the influence of serum sodium and dialysate sodium on mortality among 11,555 patients receiving dialysis across 12 countries, demonstrated that lower pre-dialysis sodium and lower dialysate sodium (<137 mmol/L) were associated with a higher mortality incidence.15 A post hoc analysis of the DOPPS trial found that the routine use of sodium profiling (loading of sodium towards the end of dialysis in order to limit intradialytic hypotension) resulted in a 36% increase in all causes of mortality (hazard ratio [HR]: 1.36; 99% confidence interval [CI]), and a 34% increase in cardiovascular death (HR: 1.34; 99% CI).16 It was postulated that sodium loading resulted in a net sodium gain, culminating in increased thirst, IDWG, and hypertension.
The paucity of RCTs means that the question of dialysate sodium reduction as a means of limiting hypertension in patients receiving dialysis remains unanswered. However, this clinical equipoise is the subject of the ongoing and much anticipated SoLID and RESOLVE trials.13,17 These multicentred RCTs will seek to provide concrete evidence as to whether the benefits of low dialysate sodium on interdialytic haemodynamics and LVMI outweigh the deleterious effects of an increased propensity towards intradialytic hypotension.
DRY WEIGHT PROBING
Dry weight is defined as the lowest achieved post-dialysis weight without the occurrence of significant signs and symptoms of hypovolaemia or hypervolaemia.18 A number of studies have shown the importance of achieving dry weight in controlling hypertension in ESKD.19,20 Extended-duration home haemodialysis (HD) has been shown to achieve satisfactory BP control in the majority of patients without the need for pharmacotherapy. This is largely due to optimal volume control, although factors such as decreased sympathetic activation may also be at play. These early studies have been validated by the more contemporary DRIP trial.21 This RCT, involving 150 patients on HD, assessed the influence of dry weight probing versus usual care. One hundred patients were randomised to the ultrafiltration arm achieved a 1 kg reduction in post-dialysis weight at 8 weeks, following a gradual reduction of dry weight. This resulted in a corresponding reduction of ambulatory BP by 6.6/3.3 mmHg compared to the 50 patients randomised to the usual care group.
Therefore, adequate volume control by dry weight probing is one of the pertinent aspects in managing hypertension in ESKD. Agarwal et al. demonstrated that LVMI, a strong prognostic marker for mortality, can be improved with dry weight probing among patients who are on hypertensive dialysis.22 This study analysed the echocardiographic parameters of the patients included in the DRIP study. LVMI fell by 6.3 g/m2 over 8 weeks in patients randomised to receive ultrafiltration compared to a 0.3 g/m2 progression seen in controls. During a post hoc analysis, the HDPAL investigators found that the treatment-mediated decline in LVMI was mitigated when adjusted for systolic blood pressure and inferior vena cava diameter (surrogate markers for volume status).23 This highlighted that improvements in LVMI were volume-related. An analysis of the DOPPS data suggested that centre-specific practices relating to the management of volume status influence patient outcomes. Centres with a protocol outlining the frequency of dry weight assessment resulted in a reduction in cardiovascular and all causes of mortality (HR: 0.72–0.78; 99% CI: 0.55–0.95 and 0.64–0.94, respectively).16
PHARMACOTHERAPY
Hypervolaemia is not the conditio sine qua non of hypertension among patients receiving dialysis.24 One study, involving more than 500 patients receiving dialysis in Europe, demonstrated that 13% of patients were hypertensive but euvolaemic, which emphasises the existence of alternative pathophysiological mechanisms behind hypertension in ESKD. Several meta-analyses have conclusively shown that pharmacotherapy in ESKD improves all causes of mortality.25,26 However, no specific class of antihypertensive agent has been clearly shown to improve prognosis over another. No study has been powered to observe a difference between classes of antihypertensive agents, nor have there been any head-to-head trials to elucidate the optimal antihypertensive in ESRD.
ACE Inhibitors and Angiotensin Receptor Blockers
Aberrant RAAS activity has been implicated in the propagation of hypertension and cardiovascular morbidity in ESKD.27 Studies have shown that the RAAS system is twice as active in ESKD compared to healthy controls. Angiotensin II, the major effector molecule of the RAAS system, activates growth factors, elicits myocyte hypertrophy, and fibroblast proliferation (via aldosterone), culminating in left ventricular hypertrophy and fibrosis.28 Outcome data pertaining to the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB) in patients on dialysis has yielded mixed results. The FOSIDIAL trial, conducted in patients who are hypertensive and receiving HD, who also have left ventricular hypertrophy, found that fosinopril did not reduce major adverse cardiovascular events or mortality compared with placebo.29 Similarly, the HDPAL trial showed that lisinopril and atenolol were equivalent in reducing LVMI.30 However, lisinopril was associated with a greater incidence of MACE, which ultimately resulted in the premature termination of the trial. ARBs may have a more beneficial effect on this population. Losartan, valsartan, and candesartan have all been associated with reduced cardiovascular events and mortality in patients on HD.31,32 Suzuki et al. reported that ARB therapy was associated with a 49% decrease in MACE and a 36% reduction in all cause-mortality in patients on HD.32 Shireman et al. suggest that ARBs may be superior to ACE inhibitors, which tend to have a higher dialysability.33,34 However, a meta-analysis by Tai et al. found that, while ACE-I/ARB therapy reduced LV mass in patients on HD, neither agent significantly improved cardiovascular morbidity or mortality.35
During the multicentred STOP ACEi RCT, 62% and 56% of patients in the discontinuation and continuation arms, respectively, progressed to ESRD. The average BP in the discontinuation arm was higher than the continuation arm. However, the difference was not seen after 15 months of the trial. Notably, the secondary outcomes of cardiovascular events, hospitalisations, and mortality were similar between the two groups.36
Despite the cardioprotective effect of ACE inhibitor/ARBs and their apparently favourable impact on LVMI, their use is often limited by their potentiation of hyperkalaemia.29 However, studies suggest that ACE/ARB therapy may be safe in patients on maintenance dialysis where potassium homeostasis is largely maintained by HD.37 One RCT analysed the treatment effect of ACE inhibitors, ARBs, and combined RAAS inhibition versus placebo on serum potassium. Of the 62 patients who completed this 3-month trial, none had to discontinue therapy due to hyperkalaemia or another adverse event (Table 1). The serum potassium was equivalent in all groups, with no statistical difference between the rates of severe hyperkalaemia.
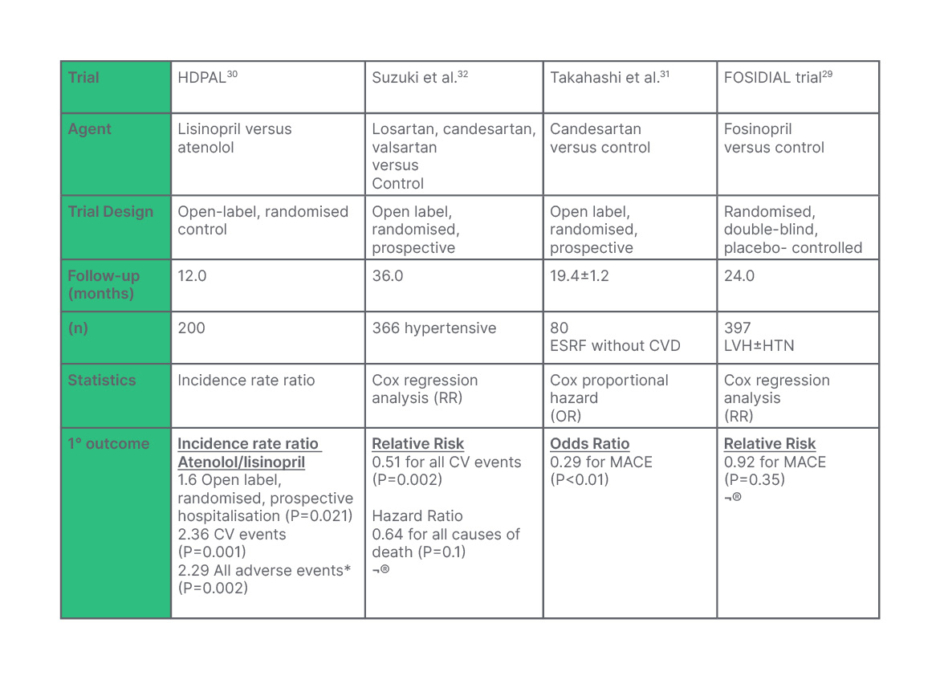
Table 1: A summary of the results of a number of trials looking at the impact of angiotensin-converting enzyme (ACE)/angiotensin II receptor blockers (ARB) therapy in patients on maintenance dialysis.
A number of these trials imply a cardiovascular benefit to ACE/ARB therapy in end-stage renal disease, whereas the HDPAL trial suggests that β-blockade may be more efficacious in reducing cardiovascular morbidity.
*All adverse events: Combined stroke, MI, heart failure, and CV death.
MACE: major adverse cardiovascular events including heart failure, unstable angina, severe arrhythmia, and cardiovascular death.
¨: No statistical difference
CV: cardiovascular; CVD: cardiovascular disease; ESRD: end-stage renal disease; ESRF: end-stage renal failure; HTN: hypertension; LVH: left ventricular hypertrophy; MACE: major adverse cardiovascular events; MI: myocardial infarction; OR: odds ratio; RR: relative risk.
While these results may seem promising, the highly regulated environment of RCTs makes it difficult to extrapolate the true safety to the wider population.38 Juurlink et al. observed an increase in hyperkalaemia morbidity and mortality (8.6 and 1.7 per 1000, respectively; P=-<0.001) after the publication of the RALE trial.38 Furthermore, the HDPAL trial revealed a 3.4-fold increase in hyperkalaemia among the lisinopril arm.30 More conclusive data encompassing larger, well-powered RCT are required to determine the true treatment effect of ACE/ARB therapy, and whether the benefits outweigh the risks of hyperkalaemia.
Calcium Channel Blockers
Dihydropyridine calcium channel blockers are in widespread use for the management of hypertension in patients on dialysis.39 One retrospective cohort study involving over 5,500 patients receiving dialysis demonstrated that dihydropyridine versus non-dihydropyridine calcium channel blockers were associated with improved mortality and cardiovascular morbidity outcomes (adjusted HR: 0.77 and 0.86, respectively). The most significant study examining the influence of CCBs in ESRD was concluded in 2008.40 This RCT, involving 251 patients on hypertensive dialysis, analysed the effects of 10 mg amlodipine on mortality and cardiovascular outcomes in ESRD. Twelve percent of patients assigned to the amlodipine group compared to 17% of the placebo arm over the 19-month follow-up had a primary end point of all causes of mortality; 15% versus 25% achieved a secondary outcome of composite all-cause mortality, cardiovascular event, stroke, or acute limb ischaemia requiring intervention (HR: 0.53; 95% CI: 0.31–0.93; P=0.03). Although all causes of mortality alone were not statistically significant, amlodipine was shown to significantly reduce the composite outcomes of mortality and vascular events.
β-Blockers
In parallel with RAAS inhibitors, β-blockers have also demonstrated cardioprotective properties.41 The HDPAL trial is the largest head-to-head study between ARB and β-blocker therapy in ESKD.30 This study concluded that each agent had comparable antihypertensive effects, but thrice-weekly atenolol was associated with lower cardiovascular morbidity and all-cause hospitalisations. This apparent cardiovascular protection may be attributed to improved aortic stiffness, as demonstrated by the secondary analysis of the HDPAL trial.42
In addition to antihypertensive properties, β-blockers have a role in the protection against fatal arrhythmia and sudden death.43 Analysis of the DOPPS study revealed the incidence of sudden death to be as high as 33%, and inclusion of β-blockers were associated with a lower incidence of sudden death (HR: 0.88; 95% CI: 0.78–0.99). Another prospective placebo-controlled trial demonstrated strong evidence for the use of β-blockers in a subpopulation of patients on dialysis with dilated cardiomyopathy.44 This trial, involving 114 patients, demonstrated a significant mortality benefit following carvedilol therapy with 51% mortality in the treatment arm compared with 73% in the placebo group (HR: 0.51; 95% CI: 0.32–0.82). This was associated with a reduction in maladaptive cardiac remodelling, and improved ejection fraction.
The challenge of studying hypertension in patients on dialysis was demonstrated by the BLOCADE trial.45 This trial examined the treatment effect of 25 mg BD carvedilol versus placebo. Of 354 eligible patients, 91 consented, and only 49 completed the trial. The major reasons for non-inclusion were clinical instability and current β-blockade. Although large-scale highly powered studies are required to elucidate the most efficacious antihypertensive and cardioprotective agents in ESKD, the experience of the BLOCADE investigators highlights the complexity of establishing such a trial. It may require multicentre international collaboration to answer such questions.
Others (Mineralocorticoid Receptor Antagonists, Hydralazine and Nitrates, and Loop Diuretics)
Numerous studies support the use of mineralocorticoid receptor antagonists in ESRD.46 The DOHAS trial demonstrated that a low dose spironolactone resulted in a 60% decline in cardiovascular/cerebrovascular events over a 3-year period (HR:0.40; 95% CI: 0.20–0.81). The RALES study demonstrated a low incidence of hyperkalaemia (2%) among study participants.47 However, subsequent population-based time-series analysis demonstrated a significant increase in hyperkalaemia rates and mortality following this publication.38 Whether the benefits of mineralocorticoid receptor antagonists outweigh the risks is the subject of the much-anticipated ALCHEMIST trial.48
Hydralazine-isosorbide dinitrate (HY-ISD) may also improve cardiovascular outcomes in ESRD.49,51 The HIDE trial concluded that HY-ISD is safe and tolerable in ESRD.49 A large retrospective study demonstrated a significant improvement in cardiovascular, as well as all causes of mortality with HY-ISD compared to placebo.50
The contribution of extracellular volume overload in the propagation of hypertension in ESRD has been well established.21 The addition of low-dose diuretics in patients on dialysis with residual renal function can offer additional improvements in natriuresis and volume management.51 Additionally, they may help maintain urine output in patients on peritoneal and HD, respectively.51,52 The influence of diuretics on patients receiving hypertensive dialysis requires further studies.
DIAGNOSIS OF HYPERTENSION IN DIALYSIS PATIENTS
Numerous studies have suggested a U-shape associated between pre-dialysis BP and mortality in ESKD.53 Adequately detecting hypertension is therefore of paramount importance in improving mortality and avoiding harm along the BP spectrum. Recently, however, a lot of focus has shifted towards the setting in which hypertension is diagnosed.54
Conventional peri-dialytic BP to diagnose hypertension has several caveats.55,56 BP variation is common in ESKD, and is closely linked to variations in volume status in the inter- and intradialytic period.57 Furthermore, one cross sectional study involving 270 patients on dialysis across seven centres demonstrated a significant elevation in pre- and post-dialysis BP (14.3/7 and 13.6/4.4; P=<0.05) when measured via conventional means compared to standard protocol. Secondary analysis of the CLIMB study demonstrated that IDWG was closely associated with BP, further supporting the role of volume in BP variation.58 Therefore, the use of peri-dialytic BP may not adequately reflect BP in the interdialytic period. Pre- and post-dialysis BP recordings are used as a parameter to reflect cardiovascular stability during dialysis, as opposed to diagnosing and assessing the response of antihypertensive medication.56 Medications are often held pre-dialysis to prevent intra-dialytic hypotension. The TAKE HOLD trial, which randomised patients on dialysis to take or hold antihypertensive agents with multiple daily dosing, demonstrated a greater propensity for pre-dialysis hypertension in the HOLD arm as part of their secondary outcome analysis; this may suggest a deleterious effect of holding such medications.59 The strongest evidence against the utility of peri-dialytic BP was provided by a meta-analysis, which looked at 18 studies involving 692 patients on dialysis.55 This study reported poor concordance between peri-dialytic BP and 44 hour ambulatory blood pressure monitor (ABPM). Numerous studies have also shown poor correlation between BP recorded on dialysis and mortality outcomes.60
Relying on peridialytic haemodynamics may also exacerbate hypertension in ESRD. Intradialytic hypotension is a frequent occurrence in this population.61 A common strategy is to withhold antihypertensives to mitigate the risk of intradialytic hypotension.62 There is no clear evidence that this strategy accurately mitigates the risk of intradialytic hypotension. Moreover, this may exacerbate interdialytic hypertension and precipitate intradialytic hypertension, which has a strong association with cardiovascular morbidity.63,64
As with patients who are not receiving dialysis, ABPM appears to be the most accurate and thus gold standard in diagnosing hypertension in dialysis.65 The advantages of ABPM include the elimination of white coat hypertension, session-to-session variability in IDWG, and the ability to obtain a high number of readings. Furthermore, ABPM has been shown to predict end-organ damage.66 One cross-sectional analysis of 140 patients on dialysis demonstrated that ABPM, unlike dialytic BP, closely approximated LVMI, a marker associated with cardiovascular morbidity and mortality. A unique function of ABPM is the ability to detect nocturnal BP. Dysregulation of the circadian rhythm of BP is increasingly common as renal function declines, and is a function of impaired ultrafiltration or excessive tubular absorption of sodium.67 There is growing evidence to suggest that ‘non-dipping’ nocturnal BP is associated with accelerated end-organ damage, the detection of which is greatly enhanced with ABPM.68
One of the major disadvantages of ABPM is the lack of availability and timely access to monitoring. With this in mind, one cross-sectional study analysed the utility of home (inter-dialytic) BP monitoring in the diagnosis of hypertension in ESKD.69 This study demonstrated that home BP measurements were 84.1% sensitive and 80% specific in the diagnosis of hypertension. Moreover, home BP has been shown to be more reproducible than ABPM. Most strikingly, one prospective cohort study involving more than 150 patients on dialysis demonstrated that home BP was accurate in determining risk from cardiovascular death.3 During this trial, patients were assigned to home BP, ABPM, or standardised and routine dialysis BP monitoring. After the 24-month follow-up period, only the home BP and ABPM groups showed correlation with cardiovascular mortality. Although notably ABPM was of more prognostic value, home BP readings showed that each standard deviation increase in BP corresponded to a 35% increase in cardiovascular mortality (HR: 1.35; 95% CI: 0.99–1.84). This demonstrates the utility of home BP where ABPM is unavailable (Figure 1).
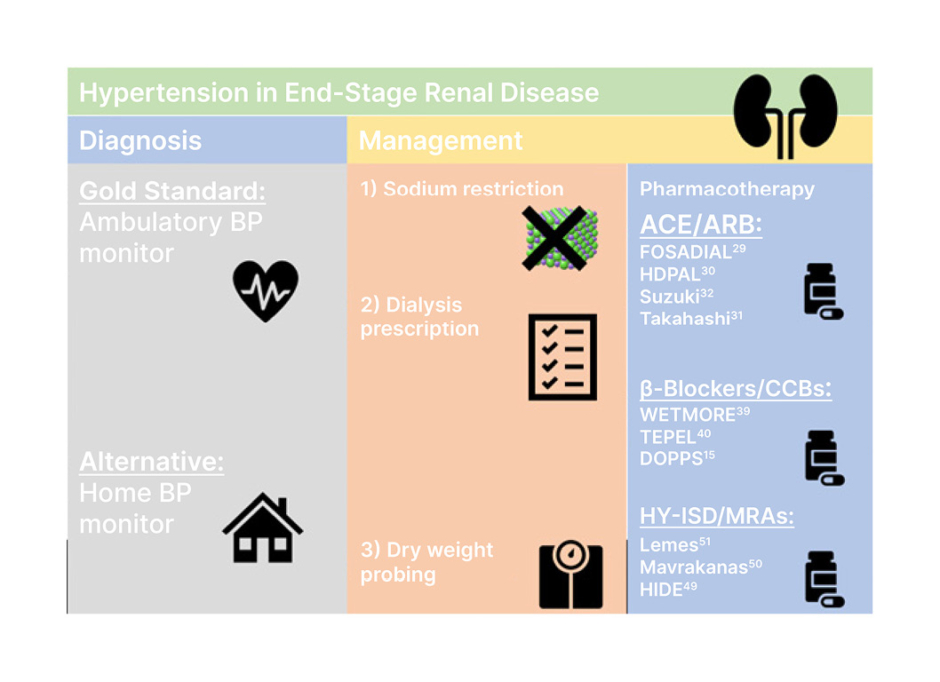
Figure 1: Summary of diagnosis and management of hypertension in end-stage renal disease.
ACE: angiotensin-converting enzyme; ARB: angiotensin II receptor blockers; BP: blood pressure; CCB: ; HY-ISD: hydralazine-isosorbide dinitrate; MRA: magnetic resonance angiography.
HYPERTENSION AND OUTCOMES IN ESKD
How does adequate diagnosis and management of hypertension translate to cardiovascular outcomes and mortality in ESKD? Early epidemiological studies showed a ‘U’-shaped correlation, suggesting that exceeding BP targets may be harmful.53 Lack of power among RCTs has made it difficult to establish the true effect of hypertension in ESKD.31,38,44,70 However, recent meta-analyses have shown clear cardiovascular and mortality benefits with antihypertensive therapy.25,26 The culmination of data from eight and five trials, respectively, have shown that antihypertensive therapy significantly reduces the number of cardiovascular events. Table 2 summarises the findings of these meta-analyses. These meta-analyses have shown that, ultimately, antihypertensive therapy improves cardiovascular mortality and all causes of mortality (HR: 0.71 (0.50-0.99) and 0.8 (0.66-0.96), respectively).
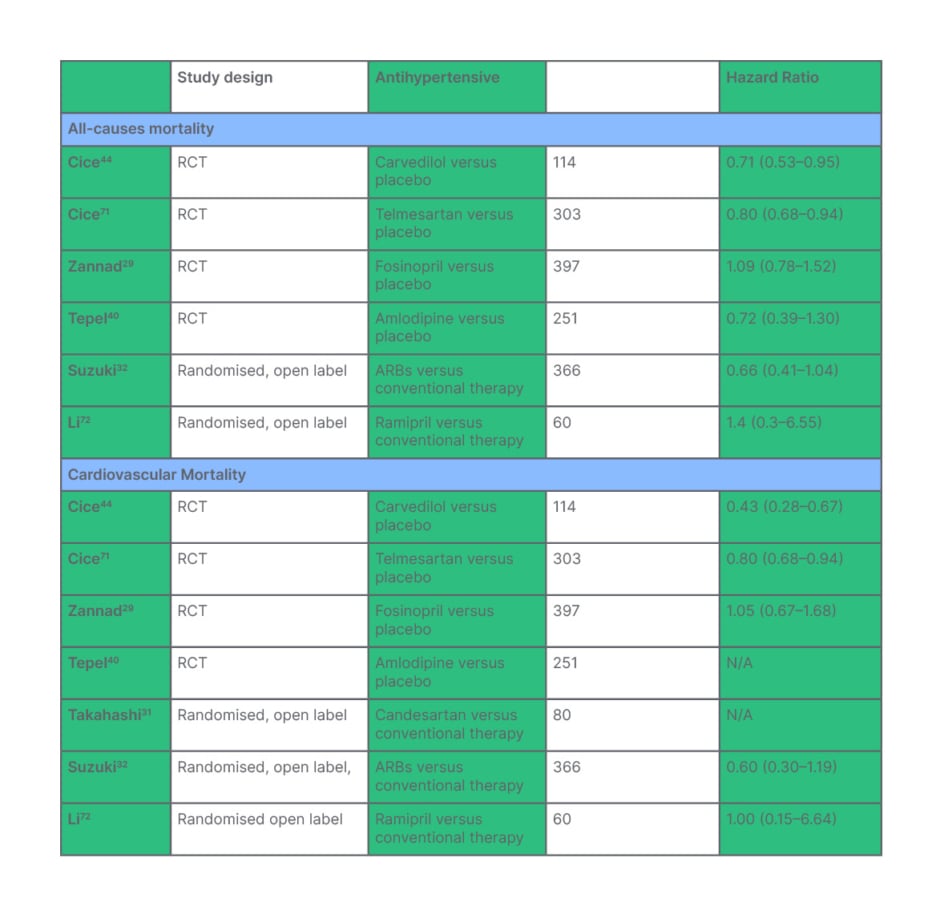
Table 2: Treatment effect of antihypertensive therapy on all causes of mortality in end-stage kidney disease is demonstrated in the top half of the table. Treatment effect of antihypertensive therapy on cardiovascular mortality in end-stage kidney disease is demonstrated in the bottom half of the table.
*N/A: insufficient data published to record hazard ratio.
RCT: randomised control trial.
CONCLUSION
In conclusion, the diagnosis and management of hypertension in ESKD is more complex than for the general population. This is, in part, because of greater variability in BP due to fluxes in volume status and renal sodium handling.58,67 ABPM appears to confer the best prognostic information, but home BP monitoring can be a useful surrogate where resources are limited.3 Different pathophysiological determinants dictate hypertension in ESKD, with volume overload playing a major role.30 Therefore, non-pharmacological interventions such as dietary sodium restriction, extending dialysis time, and altering the dialysis prescription to improve serum sodium diffusion can aid in the management of hypertension.4,14 Where adequate volume control proves ineffective in managing hypertension, antihypertensive pharmacotherapy is advised.25,26 Although numerous meta-analyses demonstrate survival benefit with therapy, no trial has demonstrated a clear benefit of one class of antihypertensive over another. Large head-to-head trials will be required to elucidate the optimal medical management of hypertension in ESKD.







