Abstract
Acute kidney injury (AKI) occurs in up to 50% of patients admitted to the intensive care unit and is associated with increased mortality. Currently, there is no effective pharmacotherapy for prevention or treatment of AKI. In animal models of sepsis and ischaemia-reperfusion, α2-agonists like dexmedetomidine (DEX) exhibit anti-inflammatory properties and experimental data indicate a potential protective effect of DEX on renal function. However, clinical trials have yielded inconsistent results in critically ill patients. This review discusses the pathophysiological mechanisms involved in AKI, the renal effects of DEX in various intensive care unit-related conditions, and summarises the available literature addressing the use of DEX for the prevention of AKI.
INTRODUCTION
Acute kidney injury (AKI) is defined by a rapid rise in serum creatinine, a drop in urine output, or both, and occurs in up to 15% of patients who are hospitalised and up to 50% of patients admitted to the intensive care unit (ICU).1 Major surgery, cardiac surgery, sepsis, cardiorenal, and hepatorenal syndrome are among the most frequent risk factors in critically ill patients. AKI is often part of a syndrome rather than a single pathophysiological entity, and the pathophysiology varies according to the underlying causes and pre-existing conditions. Renal hypoperfusion can occur due to hypovolaemia, systemic vasodilatation, increased vascular resistance, cardiac dysfunction, or increased intra-abdominal pressure leading to venous congestion. Renal hypoperfusion activates adaptive mechanisms such as vascular autoregulation and stimulation of the sympathetic nervous system and the renin-angiotensin-aldosterone system to maintain glomerular filtration rate (GFR). With prolonged hypoperfusion or inadequate adaptive mechanisms, GFR initially drops without structural damage to the renal parenchyma. However, ischaemic acute tubular necrosis occurs if renal perfusion remains compromised. Likewise, nephrotoxic drugs and endogenous toxins like myoglobin and uric acid can have a direct cytotoxic effect, compromise intrarenal haemodynamics, and cause precipitation of crystals or metabolites.2 Nearly two-thirds of AKI cases resolve within a week and in such patients, 12-month survival is over 90%. However, if AKI does not resolve, hospital mortality is significantly increased (47%) and 12-month survival is only 77%.3 Currently, there is no effective pharmacotherapy for prevention or treatment of AKI. Prevention bundles emphasise risk stratification and avoidance of hypotension, hypoperfusion, and refrainment from nephrotoxic substances.4
Dexmedetomidine (DEX) is a centrally acting, highly-selective α2-adrenergic agonist and has become an increasingly popular sedative agent in critical care due to its sedative, anxiolytic, sympatholytic, and analgesic-sparing effects, with minimal depression of the respiratory drive.5 Side effects comprise hypertension, hypotension, bradycardia resulting from vasoconstriction, sympatholytic effects, and baroreflex-induced parasympathetic activation.6,7
DEX is rapidly distributed and is mainly hepatically metabolised into inactive metabolites by glucuronidation and hydroxylation. Compared with classic sedatives like propofol and benzodiazepines, DEX provides lighter levels of sedation and supplemental analgesic effects.8 Patients remain easily rousable with minimal influence on respiratory drive. Moreover, DEX attenuates stress responses, creating a more stable haemodynamic profile during stressful events such as surgery or anaesthetic induction.9-12 Finally, DEX improves sleep efficiency and quality.13-14
The main advantage of DEX in patients in the ICU is reduction in the incidence of delirium15 and duration of mechanical ventilation.16 The use of DEX has been recommended over benzodiazepines in patients who are mechanically ventilated as it may be associated with improved outcomes.8,17-19 Clinical trials have also demonstrated that DEX-based sedation provides some advantages over usual care, typically with propofol, lorazepam, or midazolam. These advantages include a reduction in the duration of sedation and ICU stay and a possible effect on reducing the duration of delirium.8,20-22 The Maximizing Efficacy of Targeted Sedation and Reducing Neurological Dysfunction (MENDS) trial compared DEX with lorazepam.21 Sedation with DEX resulted in more time at the targeted level of sedation and more days alive without delirium or coma (median days: 7.0 versus 3.0; p=0.01) and a lower prevalence of coma (63% versus 92%; p<0.001). The Safety and Efficacy of Dexmedetomidine Compared with Midazolam (SEDCOM) trial compared DEX to midazolam.22 The prevalence of delirium was 54% in the DEX group versus 76.6% in patients treated with midazolam, for a difference of 22.6% (95% confidence interval: 14–33%; p<0.001). Median time to extubation was 1.9 days shorter in the DEX group (3.7 days versus 5.6 days; p=0.01). The multicentre, double-blind, placebo-controlled Dexmedetomidine versus Midazolam or Propofol for Sedation During Prolonged Mechanical Ventilation (MIDEX and PRODEX trials),8 compared DEX with midazolam and propofol and demonstrated the safety and non-inferiority of DEX as a first-line sedative in patients who were critically ill and on ventilation. Median duration of ventilation was shorter with DEX (123 hours; interquartile range [IQR]: 67–337]) versus midazolam (164 hours; IQR: 92–380; p=0.03) but not with DEX (97 hours; IQR: 45–257) versus propofol (118 hours; IQR: 48–327; p=0.24).8 Finally, the pivotal Sedation Practice in Intensive Care Evaluation (SPICE III) trial23 randomised 4,000 patients to receive either DEX as the sole or primary sedative or to receive usual care (propofol, midazolam, or other sedatives). Sedation with DEX did not affect overall mortality or mortality in key clinical predefined subgroups. However, it showed statistically significant heterogeneity of treatment effect according to age: DEX-based sedation appeared to increase 90-day mortality in patients below the median age of 63.7 years (relative risk increase of 23.7%) and to decrease mortality in patients older than the median trial age (relative risk reduction of 11%).24
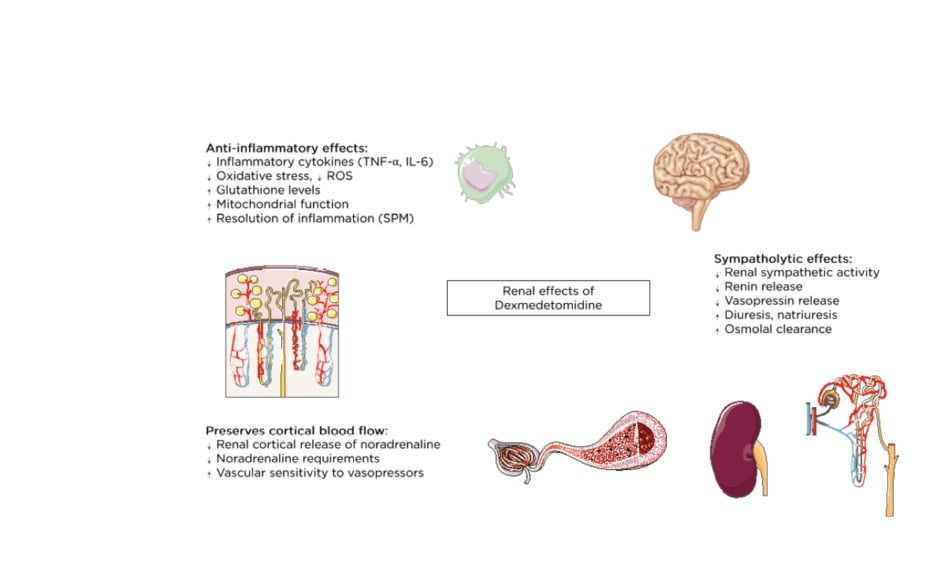
α2-agonists like DEX exhibit anti-inflammatory properties and have been ascribed respiratory, cardiac, neurologic, and renal protective effects.25 Moreover, DEX inhibits the antidiuretic action of vasopressin,26 enhances osmolal clearance, and preserves cortical blood flow by decreasing renal cortical release of noradrenaline (Figure 1).27 However, the relevance and effect of these effects on clinical outcomes remains uncertain. In this narrative review the authors address the putative reno-protective effects of DEX and summarise the results from clinical and animal studies addressing the use of DEX for the prevention of AKI.
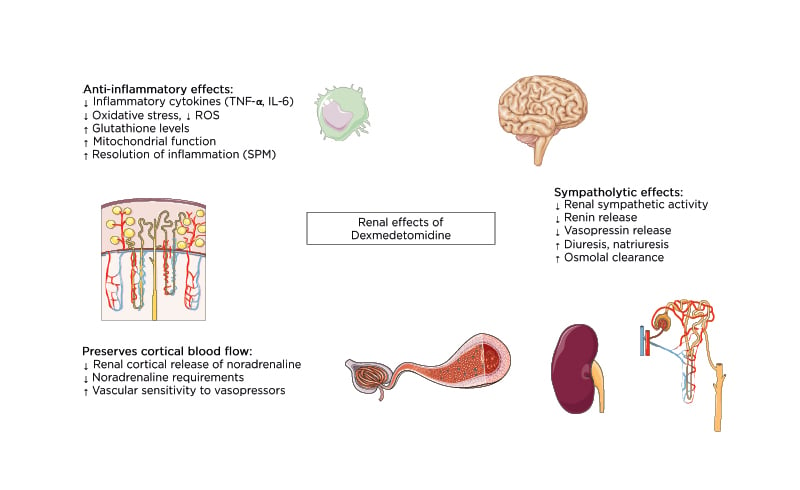
Figure 1: Overview of the renal effects of dexmedetomidine.
ROS: reactive oxygen species; SPM: specialised pro-resolving mediators.
THE ANTI-INFLAMMATORY EFFECTS OF DEXMEDETOMIDINE
DEX inhibits toll-like receptor-4/NFκB pathway activation and therefore decreases the production of proinflammatory cytokines such as TNF-α and IL-6.28-30 These actions are mainly mediated by α2-adrenergic receptor subtypes29,31 although other adrenergic-receptor-independent mechanisms,28 vagomimetic, and humoral pathways contribute to the anti-inflammatory effect.32-34 DEX also reduces oxidative stress by attenuating the formation of reactive oxygen species, increasing glutathione levels, inhibiting oxygen consumption, and improving mitochondrial dysfunction.35,36 Finally, DEX has been reported to promote resolution of inflammation through activation of so-called specialised pro-resolving lipid mediators.37 Among these, lipoxin A4 is one of the most important, and its biosynthesis depends on 5-lipoxygenase and adrenergic receptor activity. Lipoxygenase-5 and lipoxin A4 expression are increased in DEX-treated animals with sepsis, providing evidence that DEX not only inhibits the generation of excessive inflammation but also enhances its resolution.38
DEXMEDETOMIDINE FOR THE PREVENTION OF ACUTE KIDNEY INJURY IN CARDIAC SURGERY
AKI affects up to 30% of patients undergoing cardiopulmonary bypass (CPB) surgery and is the second most common cause of AKI in the ICU.39 Patients undergoing cardiac surgery are particularly at risk, as factors like non-pulsatile perfusion during CPB, hypothermia, coagulopathy, haemolysis, activation of cytokines, complement pathways and the renin-angiotensin-aldosterone system, and pituitary secretion of arginine-vasopressin in response to low-flow states result in microcirculatory and renal vasoconstriction.
Moreover, release of aortic cross-clamping leads to reperfusion injury and further cellular damage.1
Clinical and animal studies point towards a protective effect of DEX against AKI in this setting. In rodent models of ischaemia-reperfusion (I-R), intraperitoneal administration of DEX at doses between 10 and 100 µg/kg reduced inflammation and histomorphological signs of renal injury.31,34,40-45 However, these protective effects could not be replicated in studies where DEX was given intravenously (dose range: 1–3 µg/kg/hour).46,47 Nevertheless, in patients undergoing cardiac surgery, DEX appears to decrease the incidence of postoperative AKI. Several clinical trials have assessed the effect of DEX in this patient population, and DEX improves traditional48 and modern renal biomarkers49,50 and renal function in most studies.51,52 A meta-analysis and trial sequential analysis of nine randomised controlled trials (RCTs) with a total of 1,308 patients found robust evidence that DEX significantly reduced the incidence of AKI after cardiac surgery (risk ratio: 0.60; 95% confidence interval: 0.41–0.87; p=0.008).53 The protective effect on AKI was most evident when DEX was administered pre- or intraoperatively and in patients aged over 60 years. DEX also reduced time to extubation and incidence of delirium. There were no significant differences in other postoperative complications, urine output, length of ICU stay, and mortality. Compared to the earlier meta-analyses,54,55 summarised in Table 1,53-56 the study by Peng et al.53 used a more robust and transparent methodology. However, as the studies included in this systematic review covered nearly a decade, different definitions of AKI were used. Therefore, the effect of DEX on incidence of AKI after cardiac surgery under a common definition remains unclear.
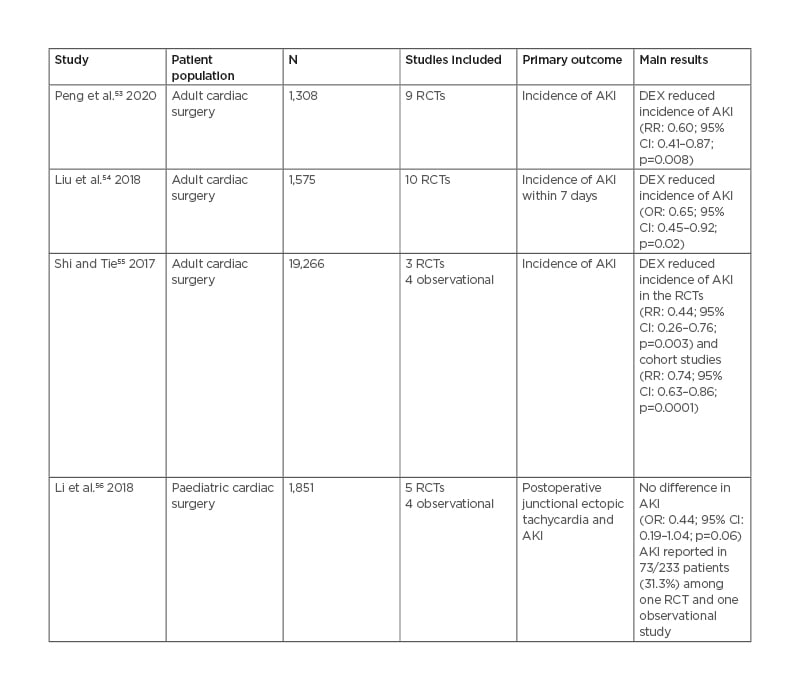
Table 1: Summary of published meta-analyses on the effect of dexmedetomidine on acute kidney injury.
AKI: acute kidney injury; CI: confidence interval; DEX: dexmedetomidine; N: number of patients; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio.
Several factors need to be considered when interpreting the results of trials and meta-analyses addressing the role of DEX for prevention of AKI. Many patients undergoing cardiac surgery have pre-existent renal dysfunction or comorbidities that make the kidneys more vulnerable to injury. As outlined above, the CPB procedure itself, aortic cross-clamping time, transfusion of blood products, high doses of vasopressors, and inotropes all contribute to the development of postoperative AKI. Therefore, any baseline variability regarding these factors between trial participants may significantly undermine the value of a meta-analysis. The potential protective role of DEX in AKI can only be appreciated when timing and dose of the intervention, type and duration of surgery, patient characteristics, and perioperative therapeutic strategies are considered.
DEXMEDETOMIDINE FOR THE PREVENTION OF ACUTE KIDNEY INJURY IN NON-CARDIAC SURGERY
Postoperative AKI affects approximately one-fifth of patients after major surgery.57 Major surgery is among the most common risk factors for AKI, as it frequently implicates significant shifts in intravascular volume, transient hypotension, and the exposure to nephrotoxic substances including contrast media, antibiotics, and non-steroidal anti-inflammatory drugs. Increased levels of circulating cytokines and reactive oxygen species due to endotoxins from compromised visceral perfusion and I-R injury contribute to renal injury.58 Furthermore, advanced age and pre-existing comorbidities including diabetes, chronic renal failure, and heart failure increase the risk for developing AKI,1 and complex surgical interventions are performed in older and sicker individuals, thus increasing numbers of patients at risk.59
Experimental and clinical data on the effect of DEX on postoperative AKI in non-cardiac surgery are rare (an overview of relevant studies in humans is given in Table 2).20,60-63 In a rat model of orthotopic liver transplantation, intraperitoneal DEX (10 µg/kg) decreased blood urea nitrogen (BUN) and serum creatinine levels and reduced histopathological kidney injury.29 However, in a single-centre retrospective cohort study of 1,207 patients, the use of intraoperative DEX was not associated with a decline in AKI after lung cancer surgery. A pilot RCT of 89 patients undergoing laparoscopic radical prostatectomy found that, compared to normal saline, an intravenous bolus of 1 µg/kg DEX at the start of surgery lowered the incidence of AKI and serum level of renal biomarkers like BUN, creatinine, and cystatin C.60
Table 2: Characteristics of relevant studies on the effect of dexmedetomidine on acute kidney injury in non-cardiac surgery patients. AKI: acute kidney injury; BUN: blood urea nitrogen; β2-MG: β2-microglobulin; CI: confidence interval; DEX: dexmedetomidine; eGFR: estimated glomerular filtration rate; ICU: intensive care unit; IV: intravenous; KIM-1: kidney injury molecule-1; N: number of patients; NA: not applicable; NaCl: sodium chloride; NR: not reported; OR: odds ratio; RCT: randomised controlled trial.
However, this was a small, underpowered pilot study and the overall incidence of AKI was low (4.5% in the DEX group and 13.3% in the control group), thus increasing the risk of a Type-I error. In a recent double-blind placebo-controlled RCT in 134 women undergoing caesarean section for pre-eclampsia, intravenous DEX (0.4 µg/kg/min for 10 minutes before surgery) resulted in lower β2-microglobulin, kidney injury molecule-1, and urine protein, but not in significant differences in BUN, serum creatinine, or urine output.61
DEXMEDETOMIDINE FOR THE PREVENTION OF ACUTE KIDNEY INJURY IN SEPSIS
AKI occurs in up to 50% of patients with sepsis, one-third of whom do not survive.64 Although sepsis is the most common cause of severe AKI in patients in the ICU, the exact mechanisms are still under investigation.65 An increased level of inflammatory cytokines and leukocyte activity can lead to the capillary microthrombi resulting in microvascular dysfunction. Redistribution of intrarenal blood flow due to abnormal vascular tone and shunting, renal inflammation, and oedema can decrease capillary perfusion and oxygen delivery. Sepsis-induced hypotension in addition to the microcirculatory dysfunction can further impair perfusion and oxygen delivery to the kidneys due to renal medullary tissue hypoperfusion and hypoxia.66,67 Early onset of renal medullary hypoxia and tissue ischaemia occurs hours before the development of AKI, despite elevated or unchanged renal blood flow, renal oxygen delivery, and renal cortical perfusion and oxygenation.68-70
The reno-protective effects of DEX in animal models have been related to its anti-inflammatory properties, which can attenuate sepsis-induced microcirculatory dysfunction.71 Both clonidine and DEX reduce the levels of pro-inflammatory cytokines (TNF-α and IL-6), while preserving the levels of an anti-inflammatory cytokine (IL-10) in septic sheep with AKI.72,73 In rodent models, DEX protects against AKI, although treatment was given either intraperitoneally35,72-74 or prior to sepsis.38,77-79 A single-centre clinical trial in 200 patients with sepsis found reductions in plasma inflammatory cytokines (TNF-α and IL-1), serum creatinine, and urinary injury biomarkers in patients receiving DEX (1 µg/kg bolus at ICU admission, and then 0.2–0.3 μg/kg/hour for 5 days) compared with propofol.62 However, the findings of this trial must be interpreted with caution as the primary outcome was not clearly defined, no sample size calculation was provided, the study protocol was not published a priori, and blinding and randomisation were not described.
In agreement with experimental findings, renal medullary tissue hypoxia has recently been indirectly demonstrated in humans with sepsis by measurable declines in bladder urinary oxygenation.81 Administration of noradrenaline can aggravate renal medullary ischaemia and hypoxia.68,69,82 In patients with sepsis, co-administration of DEX reduces noradrenaline requirements to attain the target blood pressure,83 an effect associated with preservation of renal medullary perfusion, renal medullary oxygenation, and kidney function.73 In the Dexmedetomidine for Sepsis in Intensive Care Unit (DESIRE) trial (N=201 patients) DEX did not significantly affect renal outcomes or 28-day mortality.20 However, a recent sub-group analysis of 104 patients with severe sepsis (Acute Physiology and Chronic Evaluation II scores of ≥23) found lower serum creatinine levels, improvements in renal Sequential Organ Failure Assessment (SOFA) sub-scores, and a decrease in 28-day mortality (22% versus 42%) in the DEX group.84
Despite the current lack of convincing clinical evidence to prove the renal benefits of DEX in patients with sepsis, data from animal studies support strategies that protect the kidneys from I-R injury.85 Although it is conceivable that DEX provides a protective effect in the evolution of AKI, its effect on long-term outcomes remains unknown. In a rat model, Liu et al.42 demonstrated that DEX improved histological signs of renal injury up to 8 weeks after renal clamping. However, most randomised clinical trials found either only a transient effect on renal parameters or provided only short-term follow up in the range of a couple of days. The DESIRE trial20 showed no difference in AKI, a secondary outcome, after 28 days and the above-mentioned sub-group analysis by Nakashima et al.84 found significantly lower serum creatinine but no difference in urinary output in the first 14 days.
CONCLUSIONS AND FUTURE DIRECTIONS
Numerous animal studies suggest a reno-protective effect of DEX after a controlled insult such as I-R injury or experimental sepsis. These effects are more reproducible with intraperitoneal injection of DEX, compared to the more clinically relevant intravenous route. In clinical practice, three meta-analyses confirm a beneficial effect of DEX on renal function in patients after cardiac surgery. However, the evidence for similar benefits in patients with sepsis or in non-cardiac surgery is less convincing. An important difference between these trials is the timing of DEX administration relative to the noxious insult. While in cardiac surgery the onset of CPB is predictable, it is impossible to predict the exact time of onset of sepsis, massive blood loss, or hypotension. Importantly, due to its sympatholytic properties, DEX may aggravate haemodynamic instability, raising concern for additional renal hypoperfusion with its use. However, in their systematic review, Peng et al.53 did not find any differences in hypotension or bradycardia or the need for vasopressors with DEX use, and, in patients with sepsis, DEX may actually decrease vasopressor requirements.83 Moreover, a high inter-individual variability in DEX pharmacokinetics has been described, especially in patients in the ICU and body size, liver function, plasma albumin, and cardiac output all have a significant impact on DEX pharmacokinetics.5 As outlined above, any baseline variability regarding these factors among trial participants must be considered when interpreting the results of clinical trials.
The assessment of the incidence of AKI in different patient populations has been complicated by the various definitions for AKI used over time.85 The challenges in applying diagnostic criteria in the critically ill patient are considerable. If the true baseline creatinine level is not available and a ‘baseline’ creatinine level is obtained only after a significant amount of intravenous fluid has been administered, AKI diagnosis may be falsely common because the ‘baseline’ creatinine value may be falsely low due to haemodilution is these settings.86 Moreover, ongoing fluid administration can decrease serum creatinine concentration and thereby conceal loss of GFR in such patients. Finally, the widespread use of diuretics and intravenous fluids in the perioperative period may render urine output an unreliable indicator of true renal function. Future trials are needed to determine the dose and timing of DEX in improving outcomes in different patient populations, especially in patients with decreased baseline kidney function.