Abstract
Patients can present with chief complaints and symptoms that differ from the eventual diagnoses. The differences between aetiologies versus complications must be appreciated through careful evaluation and use of clinical investigations, laboratory testing, trial of hypotheses, and clinical gestalt. Herein, this article discusses the case of a 58-year old individual who presented with impaired physical functioning, malnutrition, depression, and cognitive impairment. These four symptoms are known collectively as failure to thrive, and they often portend adverse patient outcomes. The internal medicine care team initially attributed the failure to thrive to the combination of an ongoing cervicofacial infection and a pre-existing mood disorder, but its true aetiology was more complex. In the context of various physical and psychiatric health derangements, the patient displayed clear signs of a rare disorder called Cotard’s syndrome. Due to the concern of the care team and the patient’s acting medical power of attorney, the eventual working diagnosis was made, and electroconvulsive therapy and aripiprazole combination therapy initiated, resulting in significant and improved outcomes. In addition to discussing the patient’s course of care, this case report also addresses the caution inherent in prescribing medications, the evaluation of decision making capacity, and the utilisation of a medical power of attorney. The authors also present their thoughts on minimising inefficiencies in care delivery to better the patient’s health outcomes.
Key Points
1. Cotard’s delusion (CD) follows a two-hit hypothesis, where an insult challenges the identification of an individual’s body, and another fails to resolve the psychological conflict.
2. The patient in this case study had started to improve before the COVID-19 pandemic; however, social isolation and a lack of in-person physical therapy helped to worsen their mental and physical health.
3. A rare condition, most research into CD is through case studies, which the authors reviewed to summarise the efficacy of CD treatment options.
INTRODUCTION
Cotard’s delusion (CD) is a rarely described condition in which patients present with a constellation of symptoms. There are multiple types of Cotard’s syndrome: psychotic depression (depressive episodes with secondary psychotic elements), Cotard’s Type 1 (nihilistic delusions), and Cotard’s Type 2 (a mix of anxiety, depression, and delusions).1 Of these types, there also lies a progression of illness categorised by the level of severity of the psychopathological presentation. Firstly, the germination stage presents with hypochondriasis and cenesthopathy. Secondly, the blooming stage introduces nihilistic delusion and delusion of negation of one’s own body or body part(s). Lastly, the chronic stage cements the mood changes and systematic delusions of the patient.2 Herein, the authors describe a unique case of CD with sequelae of a syndrome known as Adult Onset Failure to Thrive.
PATIENT INFORMATION
The patient is a 58-year-old individual with history of hypertension, hyperlipidaemia, Type 2 diabetes, recent left cerebellar cerebrovascular accident (CVA) in January 2020, with focal frontal white matter ischaemic changes and subcortical insults in the basal ganglia, and remote history of major depressive disorder with psychotic features, for which they received inpatient treatment as a teen, likely superimposed on an evolving neurocognitive disorder of vascular aetiology (Figures 1 and 2). They presented to the emergency department with a 2-day history of right-sided facial and neck swelling, concerning for acute sialadenitis versus cellulitis with associated dysphagia and subsequent poor oral intake. CT indicated extensive oedema of the superficial and deep spaces of the right neck and blood cultures grew methicillin-susceptible Staphylococcus aureus bacteria, prompting a screening echocardiogram that showed normal systolic function and ejection fraction without signs of valvular vegetation. Per the recommendations of Oral and Maxillofacial Surgery; Ear, Nose and Throat; and Infectious Diseases departments, the internal medicine team pursued antibiotic treatment without surgical intervention.
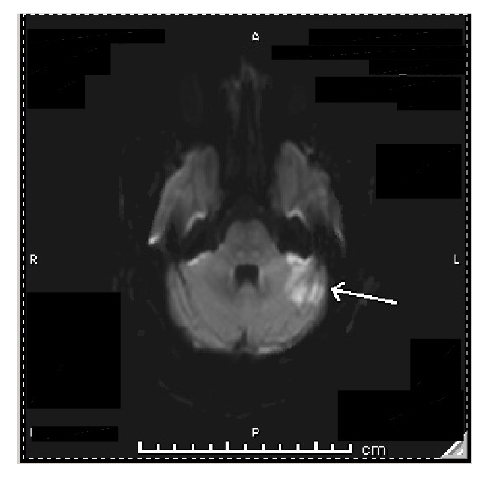
Figure 1: Axial CT images of the head from the skull base to the vertex without contrast.
Brain and bone algorithms were performed and reviewed. A prominent recent infarction involving the superior portion of the left cerebellum is associated with slightly increasing deformity of the left side of the fourth ventricle. No haemorrhage was evident. There was a potential for eventual development of hydrocephalus, although that was not present at the time. A moderate remote infarction was again seen about the anterior horn of the left lateral ventricle and basal ganglia. The brain parenchyma, ventricles, and subarachnoid spaces are otherwise unremarkable in appearance.
The patient transitioned to physical therapy in February after the CVA, and had been making progress in recovering baseline function when the COVID-19 pandemic hit. According to the patient’s children, they were an active church member, had a great appetite, and were independent in most, if not all, activities of daily living. Unfortunately, the social isolation in combination with lack of in-person physical therapy brought on by distancing measures precipitated a decline in their mental and physical health that manifested as worsening functional status.
CLINICAL FINDINGS
By the end of August 2020, the patient was bedridden and needed extensive assistance. They were neglectful of personal hygiene, socially withdrawn, and refused any oral intake, including medication and food. When the internal medicine service admitted the patient in November 2020, they had lost 70 lbs since January.
While the infection and associated dysphagia improved with intravenous antibiotic treatment, their nutritional status did not. They continued to refuse oral intake, and developed worsening psychomotor retardation, depression, and delusions, along with a severe pathological guilt perception. On multiple occasions, they disclosed that their illness was punishment for not adhering more stringently to their religious beliefs. When the care team enquired about their reluctance to eat, they simply stated that their “insides are rotting,” and that their stomach and other various organs no longer existed, a set of beliefs known as CD. At this point in treatment, they were experiencing a significant amount of anal pain and constipation (despite having consistent bowel movements), and endorsed 10 out of 10 pain according to the Numerical Rating Scale.3 They stated that a demon in the room was responsible for inflicting this abdominal pain, the presence of which invalidated their claim that they no longer had “insides” (Table 1).
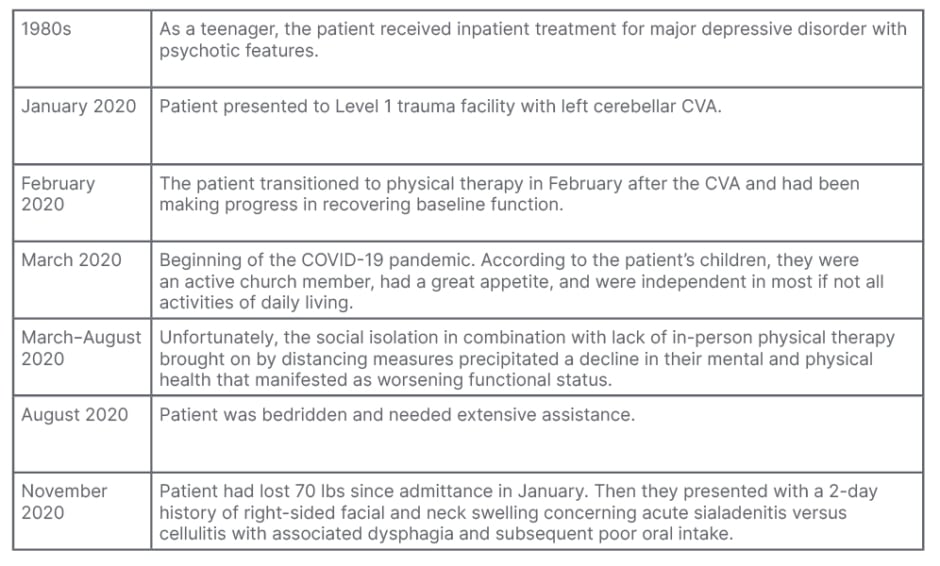
Table 1: Patient timeline.
CVA: cerebrovascular accident.
DIAGNOSTIC ASSESSMENT
With the decline in the patient’s cognitive function over the period of around 8 months, several contributory factors were considered. These include a precipitating episode of a mood disorder, substance- or medication-induced psychosis, catatonia, dementia, metabolic disorders, infection, and CD. With the patient’s history of major depressive disorder with psychotic features, an episode of such was the most likely diagnosis. Other psychiatric problems with similar presentations include catatonia, a treatable condition, and dementia, a long-term health condition caused by the degeneration of neuronal cell bodies, often diagnosed through clinical exploration.
The patient’s mental status, nevertheless, might have been affected by other derangements. The patient was taking lorazepam, a drug known for precipitating psychosis or delirium in patients, which with the cessation of the medication resolved. The adverse effect of medications along with infectious aetiologies, a well-known cause of altered mental status, and the main reason for the patient’s initial presentation, were considered and ruled out as part of the clinical investigation.
The ultimate diagnosis of CD was one of exclusion, taking into account the constellation of delusions that our patient demonstrated. An important consideration regarding this diagnosis was the sequelae of a syndrome known as adult onset failure to thrive, with which the patient clearly manifested in their initial presentation. More common in the geriatric population, especially those with chronic or existing comorbidities, failure to thrive is characterised by cognitive impairment, malnutrition, decline in physical function, and depression.4 While the deterioration in physical and nutritional health could be mistaken for symptoms of a melancholic depression with delusions or even hallucinations, the patient’s nihilistic delusions, along with the accompanying religious persecutory undertones seen in many other CD cases, make this aetiology more likely.5
THERAPEUTIC INTERVENTION
The initial approach to CD requires meticulous assessment of the patient’s psychiatric and medical features. The treatment of underlying medical or neuropsychiatric disorders should take precedent. In particular, for severe cases presenting with feeding refusal, as in this case presentation, enteral nutrition should be considered until by-mouth fluid and food intake is resumed. The patient had a Dobhoff tube (DHT) placed. Nutritional deficiencies can precipitate or exacerbate delusions and psychosis, thereby reiterating the importance of its treatment. After addressing the more life-threatening conditions, efforts should shift towards treatment of CD if symptoms have yet to resolve.
The patient was initially restarted on home medications that included olanzapine 10 mg each bedtime and sertraline 50 mg/day, albeit with little to no improvement. The patient reported continued severe depression and CDs, and they had poor oral intake even with motivational interventions. Eventually, alprazolam 0.25 mg was administered prior to meals to reduce the anticipatory anxiety from the dysphagia. Due to its significant sedative effects and precipitation of psychosis, however, they were weaned off the medication and started on electroconvulsive therapy (ECT) and aripiprazole combination therapy.
After the medical power of attorney consented to ECT treatment, their sessions occurred every Monday, Wednesday, and Friday. As ECT targets depressive symptoms, improvements in oral intake and effort were anticipated. ECT was further ideal for this patient as it is indicated with depression with psychotic features, prior treatment resistance, and poor food and fluid intake, all of which were seen in this patient. Aripiprazole was initiated prior to their first ECT appointment and was uptitrated to 10 mg/day, with which they were discharged to continue outpatient as an adjunct to the ECT. Research comparing aripiprazole to other antipsychotics suggests its poorer efficacy relative to olanzapine, but its metabolic effects and sedation are more tolerable.6 The patient, specifically, has a history of hyperlipidaemia that precludes the use of olanzapine.
OUTCOME
The patient showed a favourable response to ECT within a couple weeks after its initiation, and tolerated the treatments well. Their oral intake markedly improved and ultimately their DHT was removed. The patient’s speech rate and fluency also improved, and their social interactions started to include humour in greater frequency. They reported mild headaches and short-term memory loss after a few ECT sessions, but the symptoms self-resolved. Ultimately, the combination of aripiprazole and ECT was most effective in reducing the severity of the CD and treating the patient’s depression. The patient’s rapid and significant response to ECT treatment is consistent with prior research.
One of the central issues encountered by the care team involved the patient’s anxiety surrounding swallowing food. This anxiety had to be assuaged through the use of a benzodiazepine, which is a medication with the potential to worsen acute or chronic psychosis. This side effect occurred in the case of the patient in discussion. The third leading cause of patient morbidity and mortality is iatrogenic; therefore, ensuring that new or worsening symptoms are not caused by the treatment strategy is key to ensuring patient safety and quality of life.7 Through carefully examining the side effect profiles of different medications and weighing the need for its use, the physician cares first for the patient and works to fulfill the oath to ‘do no harm’.
The second key clinical pearl involves patient capacity and autonomy. Patient autonomy is held to the highest regard in healthcare; however, its adherence can conflict with the physician’s duty to protect their patients when they demonstrate self-neglect or self-injurious thoughts or behaviours. Decision-making capacity is especially limited in those with CD due to its characteristic nihilistic and fatalistic delusions. The delusions hampered the treatment and recovery of the patient, prompting an assessment and subsequent decision that their child step in as medical power of attorney. However, a critical point to note is that decision-making capacity is a time-based assessment that can improve or change over the course of treatment. The Decision-Making Capacity Assessment (DMCA) is the gold-standard of patient-centred care, and can be reassessed at any point in care by the physician. This rule allows for a precise and accurate representation of patient decision-making capacity on a temporal scale, which ensures that patient autonomy is respected.
The third and final key principle is systems thinking and solutions to ensure that the patient receives the best care possible. While the patient made a full recovery, the journey towards restoring their baseline health was anything but promising. Their ECT treatments were a demonstration in systemic failures and its cost to timely care. The team responsible for transporting them to the treatment facility at Seton Main Hospital, Austin, Texas, USA, was often late, although they could not control traffic, challenging patients, poor directions, or miscommunication, etc. When tardiness was not the issue, however, paperwork was. The transportation team somehow misplaced the signed consent form that accompanied their paper chart during the first trip, so the initiation of treatment was postponed until the primary care team repeated the necessary steps to get informed consent. ECT is so limited in availability that rescheduling can be very challenging. The care team tried to co-ordinate for an inpatient transfer to Seton Main Hospital to circumvent the transportation mishaps, but there were no available beds. Without any other foreseeable or permanent solutions, the care team worked with the case manager to implement workarounds. The team started to arrange for transportation to pick them up 2 hours prior to their appointments to account for the late starts, and made sure the paper charts had the consent form attached before handing it off. The team then had the patient’s child meet them at Seton Main Hospital to ensure that the documentation was still on their person.
The patient had to defer a couple other ECT sessions due to a missed order about stopping their DHT feedings. Per ECT protocol, patients are supposed to be nil by mouth 24 hours prior to treatment. This information was communicated both verbally to the night nurse and electronically in their medical chart as an order, but the DHT was sometimes on continuously up until the patient’s transport to Seton Main Hospital. It was not until they arrived at the facility that the mishap came to light. The care team found that taping a note written in large letters about when to discontinue the patient’s DHT to be most effective. Other similar strategies can and should be implemented to ensure that systems work in the benefit of the patient.
PATHOPHYSIOLOGY
Pathophysiological explanations of Cotard’s syndrome remain under investigation in the medical community, but several ties to specific psychopathological and neurobiological disease states offer clarity.8 The presentation of Cotard’s syndrome is most apparent in the psychiatric interview rather than traditional imaging, as the most profound and common clinical signs are depression and nihilistic delusion.9 In this case study, the CVA event superimposed on the patient’s history of depression and subsequent isolation were likely inciting factors.10,11 Subcortical and basal ganglia damage, as seen in the imaging herein (Figure 2), has previously been documented as probable aetiologies of Cotard’s-like delusions.12
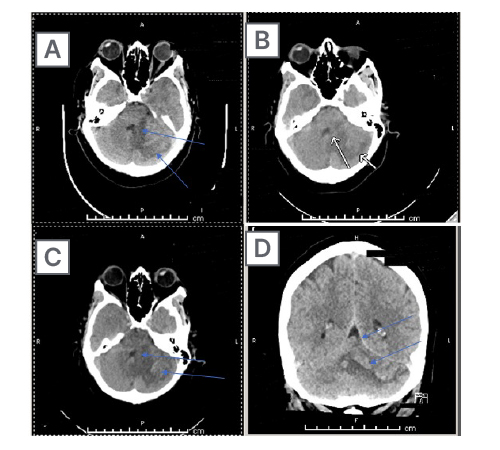
Figure 2: Axial CT images of the head from the skull base to the vertex without contrast.
Brain and bone algorithms were performed and reviewed. Hypoattenuation was again noted in the left cerebellar hemisphere, along with findings of cortical laminar necrosis and petechial type haemorrhage. Mild mass effect is again noted on the fourth ventricle, but without cephalisation. Of note are old left basal ganglia and left frontal periventricular infarctions. There was no evidence of new oedema, midline shift, new intracranial haemorrhage, or extra-axial fluid collection. An acute cortical infarction is not apparent. Demonstrated here is (A) subacute swelling and (B) subcortical damage from mass effect, all secondary to (C) the initial cerebellar stroke. The initial injury and the impingement on the fourth ventricle led to damage in the patient’s brain in the subcortical space and the basal ganglia (D).
Vascular damage to the insular cortex could also offer an organic explanation to Cotard’s syndrome. CD is believed to follow a two-hit hypothesis, where one insult can challenge the internal identification of one’s own body, and the second fails to resolve the psychological conflict when presented with evidence of the contrary. The insula allows control of introspective homeostasis, emotion, and other properties of mood. Damage here, the first hit, could lead to loss of external or internal interpretation of one’s own body, leading to the hallmark sign of nihilistic delusion, or failure to recognise one’s body.13 Additionally, damage to any of the connections of the limbic system to cortical recognition areas could lead to loss of recognition of one’s body parts, leading to the cognitive progression of nihilistic delusion.12 Ischaemic insults to frontal lobe parenchyma has led to a variety of misidentification delusions, of which pertains to CD. The current understanding is that deep vascular frontal lesions could obliterate connections from the frontal lobe with basal ganglia structures, leaving the patient unable to executively resolve the conflict of misidentification, thereby establishing the second hit. These vascular challenges are consistent with the patient presentation. This two-hit hypothesis for misidentification delusions can thus be applied to the various themes of the delusions experienced by patients, including religiously-reinforced delusions, delusions of negation, and nihilism.
DISCUSSION
Given its rarity, most research on CD are case studies. Although further research is necessary to improve the strength of the evidence behind treatment modalities, the aim of the following is to review available case studies and summarise the efficacy of pharmacological, ECT, and other treatment options used in CD.
Medications trialed in previous literature include antipsychotics, anticonvulsants, antidepressants (selective serotonin reuptake inhibitors and serotonin–norepinephrine reuptake inhibitors), and benzodiazepines. Clinical symptoms generally guide the class of medication used in treatment. When affective symptoms predominate, typically antidepressant monotherapy is first line. However, when psychotic symptoms predominate, antipsychotic monotherapy is preferred. It is important to remember to also rule out other causes of a patient’s symptoms prior to treating the patient solely on an assumption that their affective and/or symptoms are purely secondary to mental illness.
Monotherapy with antipsychotics has proven to be effective in a number of CD cases. Monotherapy with olanzapine at a dose of 5 mg/day has been successful in case reports involving younger patients who developed CD subsequent to chronic psychoactive substance use and a traumatic brain injury.14 Quetiapine at doses of 50 mg/day was shown to be efficacious in treating CD symptoms in a patient with Parkinson’s disease (PD) as well as in another patient presenting with CD in the context of multiple organ dysfunction syndrome secondary to a traumatic bone injury.15 Studies have shown a statistically significant increased risk of mortality with the use of atypical antipsychotic medications in PD, but quetiapine has the weakest association.16 Although serious consideration must be taken when prescribing antipsychotics for patients with PD and CD, quetiapine has some advantages.
Another antipsychotic used in treatment of CD is risperidone, and its use as a monotherapy was sufficient to treat several patients diagnosed with CD.17 Optimised risperidone doses ranged from 0.5 to 6.0 mg/day. Risperidone has been used in combination with other pharmacological agents as well, including valproic acid and lithium. The data suggests positive responses to treatment. A female diagnosed with postpartum depression showed signs of improvement after 2 weeks of combination therapy with 750 mg sodium valproate and 4 mg risperidone.18 In a male patient experiencing both Capgras delusion and CD, treatment with 6 mg/day risperidone and 900 mg/day lithium carbonate demonstrated some efficacy.19
Antidepressants are also effective in treating CD. Venlafaxine and duloxetine reported good responses in controlling CD when combined with various antipsychotics. In a patient diagnosed with major depression, a 4-week course of venlafaxine 150 mg/day and quetiapine 400 mg/day showed improvements in their CD.20 Duloxetine used in combination with amisulpride at doses of 60 mg/day and 400 mg/day, respectively, in an elderly individual experiencing complicated grief also demonstrated efficacy. Their delusions and depressive symptoms subsided, and they were discharged after 2 months of hospitalisation.21 Risperidone 6 mg/day combined with imipramine 150 mg/day was used in the case of a patient in their 30s with severe depression. They became asymptomatic a few months after discharge from inpatient treatment.22 Benzodiazepines have reported positive outcomes when used as an adjunct.23 In a case of a patient with CD and catatonia, lorazepam 1 mg three times daily was prescribed, along with olanzapine 15 mg/day and mirtazapine 30 mg/day.24
Evidence suggests that patients with Cotard’s are more responsive to ECT than to pharmacological treatment.25 ECT has shown efficacy when pharmacological treatment was unsuccessful in several patients, and is both well-tolerated and efficient in its treatment of CD.26 When successful, ECT demonstrated immediate results with long term symptomatic improvement. ECT can be used as first line treatment, but its availability may be limited. ECT has also been used in combination with atypical antipsychotics, including risperidone, amisulpride, and olanzapine. There were some specifically satisfactory responses to olanzapine and ECT co-administration;24 however, details of dosing for olanzapine were not reported. The concomitant use of ECT and typical antipsychotics were documented with the use of perphenazine, flupentixol, trifluoperazine, thioridazine, and haloperidol.27 In one case, ketamine was used along with ECT.28 ECT and nomifensine were paired successfully to treat CD in a medically handicapped patient.23 The use of ECT with tricyclic antidepressants was reported in severe cases of CD that presented with self-injurious behaviour, guilt and ruin delusions, and marked weight loss. In a patient who was pregnant, the combination of ECT with chlorpromazine and dothiepin led to sustained recovery.27
In a complex patient presenting with CD in the context of limbic encephalitis, olanzapine, escitalopram, and lorazepam elicited no response, so ECT was later utilised. The patient reported symptomatic relief.29 Patients who developed CD in the setting of frontotemporal dementias were treated successfully with ECT after pharmacological therapy was ineffective.30 Discontinuation of identified neurologically offending agents have had positive results in some cases. After haemodialysis was performed on two patients presenting with CD secondary to valacyclovir administration, there was complete remission of neuropsychiatric adverse effects. There were two other cases wherein the patients’ psychiatric sequelae was resolved after withdrawal of valacyclovir.Throughout the patient’s evaluation, self-injury and self-starvation should be assessed, as well as exploring the benefit of cognitive behavioural therapy and behavioural rehabilitation. Transcranial magnetic stimulation was assessed in a patient who underwent cognitive behavioural therapy had some suggested efficacy.27
CONCLUSION
As there may have been many reasons to explain the patient’s mental status, only by working through a differential diagnosis by process of elimination did it become clear that the patient had CD. Between the altered mental status, refusal of oral intake, and intense abdominal pain, the providers were confident in their diagnosis. The care team was able to successfully treat the patient with a DHT, and a combination of ECT and aripiprazole. Throughout this case study, the importance of iatrogenic causes of disease, systemic failures leading to issues with patient care, and care in the balance between what the patient wants and what is best for the person were demonstrated. These clinical pearls are vital in the building of clinical decision making, and are key when evaluating how best to care for patients.
PATIENT PERSPECTIVE
While undergoing treatment, the patient still had residual delusions, as well as newfound difficulty with memory and word fluency, known side effects of ECT therapy. The team could not accurately assess patient perspective until the complete remission of symptoms, mainly because their reasoning and decision-making capacity remained impaired. Unfortunately, the patient was discharged home to their family’s care and finished the course of ECT as an outpatient. Hospital policy at the time of the pandemic strongly recommended that patients deemed stable be discharged to conserve resources for more emergent cases, and to limit their overall risk of getting a nosocomial infection. The attempts to contact the patient after their discharge were not successful.








