Meeting Summary
Motor fluctuations (MF) are still under-recognised and under-treated in patients with Parkinson’s disease (PD). End-of-dose wearing-off is a considerable problem in the overall management of PD and is a result of the decreased therapeutic effect of levodopa/dopa-decarboxylase inhibitors (DDCI). It can be present in the early stages of PD and be difficult to recognise. During a routine neurological clinical evaluation, key questions and specific rating scales for fluctuations can be helpful to gain insights into a patient’s movements throughout their day. Wearable technology has been developed to overcome the shortfalls of frequent home diary entries for patient ON-/OFF-times, and can measure daytime variations of bradykinesia, tremor, dyskinesia, and freezing of gait. Telemedicine also provides physicians with a ‘window’ into their patients’ daily lives. Treatment decisions for newly-identified MF should consider current PD treatments, which adjunctive to add first (for levodopa/DDCI monotherapy), or which adjunctive to add next (for combination therapy). Choice of adjunctive therapies include catechol-O-methyltransferase (COMT) inhibitors, such as opicapone, monoamine oxidase-B (MAO-B) inhibitors, and dopamine agonists. Opicapone 50 mg has shown efficacy as a first-line adjunctive to levodopa/DDCI in patients with end-of-dose MF (OFF-time reduction:
68.8 minutes; ON-time increase: 79.8 minutes) versus placebo (p=0.0161 and p=0.0049, respectively), with a two-fold greater reduction in OFF-time versus placebo for both low-dose and higher-dose levodopa regimens, and significant OFF-time reductions in patients receiving <4 (-124.5 minutes; p=0.0397) or ≥4 levodopa intakes (-114.1 minutes; p=0.0001) versus placebo. Further data from four ongoing opicapone studies are eagerly anticipated.
Introduction from the Chair
Fabrizio Stocchi
Here the speakers present highlights of a virtual satellite symposium at the 7th Congress of the European Academy of Neurology – Virtual 2021, reviewing the practicalities of identifying motor fluctuations in patients with Parkinson’s disease (PD), including the use of tools and wearable technology, and considerations to meet the challenges of virtual clinics. The speakers go on to look at therapeutic options currently available for the management of MF, focussing on the role of the catechol-O-methyltransferase (COMT) inhibitor opicapone as an adjunct to levodopa/dopa-decarboxylase inhibitors (DDCI) in the management of early MF.
Identifying Motor Fluctuations: Present and Future
Fabrizio Stocchi, Mónica Kurtis, and Francesca Morgante
Levodopa remains the gold standard of symptomatic efficacy for the treatment of motor symptoms in patients with PD;1 however, as the disease progresses, patients develop motor response oscillations such as end-of-dose wearing-off and levodopa-induced dyskinesias.2,3 Wearing-off, a result of decreased therapeutic effect of levodopa/DDCI, represents a major source of disability for patients with PD, impacting on quality of life.4 Wearing-off also presents a considerable problem in the overall management of PD. Key to the timely detection and management of wearing-off is the ability to recognise that it can be present in the early stages of this disease.
Motor Fluctuations Occur Early in Parkinson’s Disease Progression
Fabrizio Stocchi presented a patient case, including the patient describing her symptoms during a routine clinical evaluation. Wearing-off was characterised by non-motor symptoms (tiredness) and motor symptoms (tremor, bradykinesia), despite the early disease stage (4 years since diagnosis) (Table 1).
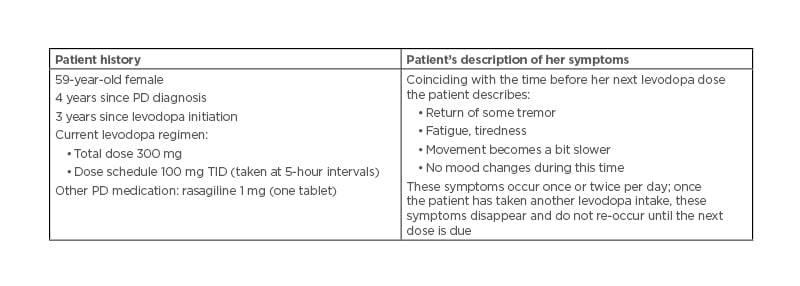
Table 1: Case study of wearing-off in early Parkinson’s disease.
PD: Parkinson’s disease; TID: three times daily.
Wearing-off is investigated during a clinical evaluation in a number of ways. DEEP, a multicentre, observational study in 617 patients with PD, showed that wearing-off during the first years of PD (2.5–5 years’ disease duration) was identified through a neurologist evaluation in 36.2% of patients and in 54.6% of patients using the Wearing-Off Questionnaire (WOQ-19).4 A recent market research survey of 420 European healthcare professionals found that MF were most frequently identified by asking the patient (82%) or hearing directly from the patient about their wearing-off symptoms (56%) (Bial, unpublished data). Only 4% of those interviewed used the WOQ-19 tool, despite one-third of healthcare professionals recognising that MF are underdiagnosed, and one-quarter feeling that MF can be hard to diagnose during a routine neurological clinical evaluation (Bial, unpublished data). These data highlight the importance of assessment techniques in the recognition of early MF during a routine neurological clinical evaluation.
Timely Identification of Motor Fluctuations
Key patient questions to identify motor fluctuations
As part of a routine clinical evaluation, a selection of key questions can help neurologists to identify MF; similar questions phrased in different ways and the use of diagrams may also help to gain insights into a patient’s movements throughout their typical day (Figure 1).
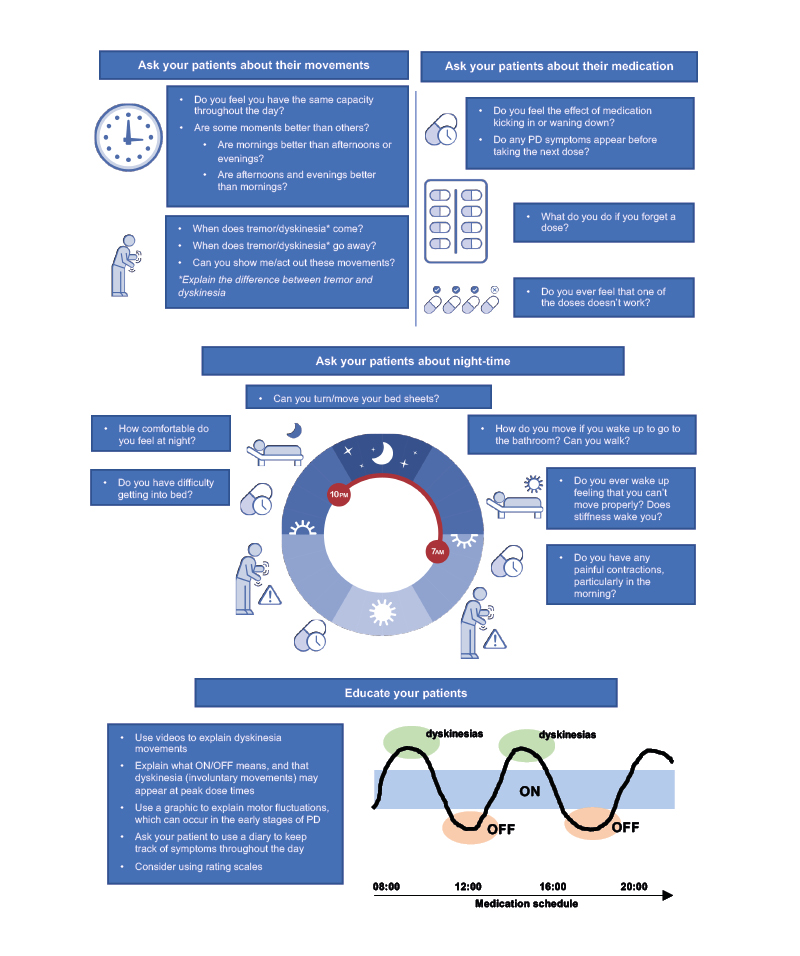
Figure 1: Key questions: identifying motor fluctuations in early Parkinson’s disease.
PD: Parkinson’s disease.
Screening and rating instruments to identify fluctuations
There are a number of rating scales for fluctuations, including UPDRS-IV, Part B;5 the Non-Motor Fluctuation Assessment (NoMoFA) Questionnaire;6 and the WOQ-19.7 The UPDRS-IV, Part B can be used to screen a patient for time spent in the OFF-state, and has advantages in that it can be used to screen for the functional impact of fluctuations and complexity of MF.5 The NoMoFA rates the severity of non-motor symptoms and whether these are worse during ON- versus OFF-time; the NoMoFA is potentially more useful in the research setting.6 The WOQ-19 is an easy-to-use questionnaire based on patient-reported outcomes and includes items for both MF and non-MF.7 Using this tool can save time during a clinic visit as it can be filled in by the patient in the waiting room before the consultation.
In the case study described, the WOQ-19 questionnaire would be a useful tool to identify both the patient’s motor and non-motor symptoms, including item 1 (tremor), item 6 (weakness), item 8 (slowness of movement), and item 5 (mood changes). However, the WOQ-19 does not offer any insights on severity of each fluctuation symptom or on sleep-related fluctuations.7
It is important to note that none of these instruments provide an understanding of fluctuation timings during the day and this information is needed to precisely adjust medication doses.
Telemedicine during the COVID-19 era: motor fluctuations on show
Telemedicine offers improvements to the quality of care8 by providing clinicians with an opportunity to ‘step into the homes of their patients’ and presenting a ‘window’ into patients’ daily lives.9 In Mónica Kurtis’ experience, observing a patient on-screen is preferable to a phone consultation. Being able to see the patient in a video call can remove the ‘performance bias’ that may occur during a routine clinic visit, enable a physician to see a patient when they are OFF (which rarely happens during a clinic visit), and offers an opportunity to pinpoint the types of spaces that can cause movement difficulties; for example, freezing of gait is often triggered when passing through narrow spaces/passages such as doorways.10 Practical tips for conducting telemedicine consultations are given in Table 2.
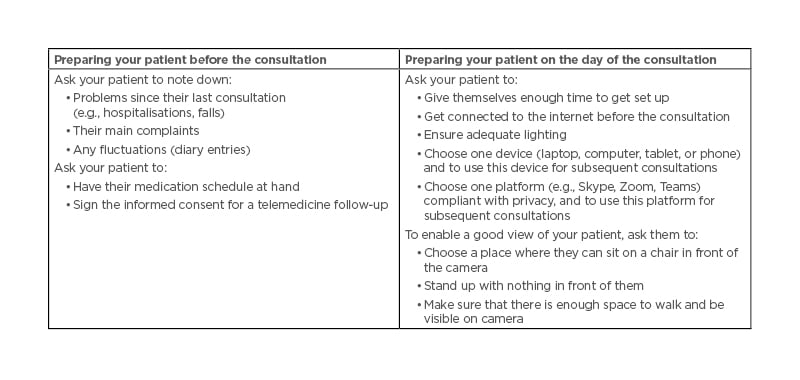
Table 2: Telemedicine practical tips for a virtual home consultation.
In the case study described (Table 1), a telemedicine consultation coinciding with the patient’s next levodopa dose may allow the physician to observe the patient’s tremor and slowness of movement.
Other advantages telemedicine can provide are an increased accessibility to movement disorder specialists, a decrease in the caregiver burden, and cost savings.8,9 There are, however, disadvantages. In Kurtis’ experience, it is not possible to assess muscle tone and even with a high-definition camera it is difficult to examine eye movements in detail. While telemedicine does limit personal contact, ties with the patient can be strengthened by meeting family members/pets and getting to know the patient’s environment. It is important to note that individual institutions and countries have their own regulations for remote consultations.
Novel technologies to detect motor fluctuations
Wearable technology, such as smartwatches or actigraphs, has been developed to overcome the shortfalls of patient ON/OFF home diaries,11 including the need for diary entries every 30 minutes; assumptions that patients know the difference between ON, OFF, and ON with troublesome dyskinesia; and the lack of distinction between different types of fluctuations.
Such technology can measure daytime variations of bradykinesia, tremor, and dyskinesia.12 Wearables are also able to detect freezing of gait,13 and have been recently shown to detect fall events.14 While these are real advances in detecting and monitoring fluctuations, the ideal wearables need to be easy to understand from the patient perspective, be eligible for reimbursement, provide measures for non-MF, and be able to record the full spectrum of night-time symptoms.
Therapeutic Options for Newly Detected Motor Fluctuations
Fabrizio Stocchi, Heinz Reichmann, and Joaquim Ferreira
Treatment Considerations for Newly Identified Motor Fluctuations
During a consultation, treatment goals should be discussed with the patient. These goals are different for individual patients and should include improving the most troublesome symptoms; for example, improving Parkinsonism symptoms, reducing OFF-time and increasing ON-time, and improving non-motor symptoms such as tremor, fatigue, and anxiety.
After considering patient factors such as age, severity of motor complications, dyskinesia, cognitive impairment, and neuropsychiatric problems,15 there are two key questions for choice of treatment for end-of-dose wearing-off: what treatments is the patient already receiving; and which treatment to add first (in the case of levodopa/DDCI monotherapy) or which treatment to add next (if the patient is already receiving adjunct therapy)? Treatment decisions for choice of adjunct class (COMT inhibitor, MAO-B inhibitor, or dopamine agonist), as well as within-class, should be based on evidence for efficacy and acceptable tolerability, as well as ease of administration and titration. However, regarding which treatment to start first, there is currently no clear-cut evidence to suggest one particular medicine over another.15
Whenever a new adjunct treatment is added, apart from providing explanations and education to the patient about MF, wearing-off, and dyskinesia, possible side effects of treatments should also be discussed. Heinz Reichmann suggested an early appointment 2–4 weeks following a new adjunct treatment being initiated to discuss side effects such as dyskinesia, dizziness, or hallucinations.
Opicapone as an Early Treatment Option for Motor Fluctuations
The clinical efficacy and safety of opicapone as an adjunct therapy to levodopa has been demonstrated in two large, Phase III, multinational, randomised, double-blind studies with open-label extension periods. BIPARK-I was an active comparator (entacapone) and placebo-controlled study (n=600), and BIPARK-II was a placebo-controlled study (n=427).16-18 In both trials, the primary endpoint was change from baseline to end of study treatment in absolute OFF-time.16,18 In BIPARK-I, treatment with opicapone 50 mg was superior to placebo (mean difference in change from baseline: -60.8 min; 95% confidence interval [CI]: -97.2 to -24.4; p=0.0015), and non-inferior to entacapone (-26.2 min; 95% CI: -63.8 to 11.4; p=0.0051 for the non-inferiority test).16 In BIPARK-II, the adjusted treatment difference versus placebo was significant for opicapone 50 mg (treatment effect: -54.3 min; 95% CI: -96.2 to -12.4; p=0.008).18 Opicapone was generally well tolerated, with the most common adverse events associated with opicapone treatment including dyskinesia, insomnia, constipation, and dry mouth.16,18
Opicapone in patients with recent and long-standing motor fluctuations
Building on these Phase III data, exploratory post hoc analyses evaluated the efficacy and safety of opicapone in levodopa/DDCI-treated patients with PD with ≤1 year duration of MF (recent motor fluctuators; RMF), as well as >1 year duration of MF (long-standing MF; LMF).19 Data from matching treatment arms in BIPARK-I and -II were combined for the placebo and opicapone 50 mg groups and analysed.19
Baseline patient characteristics, including age and daily OFF-time, were similar for opicapone (RMF: n=85; LMF: n=162) and placebo (RMF: n=71; LMF: n=174) groups in both RMF and LMF patients.19 The LMF group had a longer mean disease duration (placebo: 8.5 years; opicapone 50 mg: 8.6 years) compared to RMF (placebo: 5.8 years; opicapone 50 mg: 5.9 years),19 as well as higher mean daily levodopa dose (LMF placebo: 742.3 mg; opicapone 50 mg: 739.3 mg) than RMF (RMF placebo: 585.4 mg; opicapone 50 mg: 616.6 mg) patients.19 Remarkably, changes in absolute OFF- and ON-time were significantly greater for opicapone versus placebo in both RMF and LMF, with opicapone reducing absolute OFF-time by approximately 1 hour in both groups versus placebo (Figure 2).19
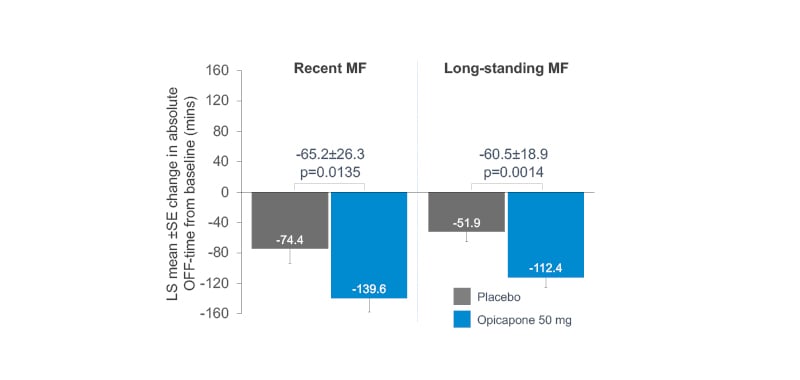
Figure 2: Mean changes in OFF-time in recent and long-standing motor fluctuators: opicapone versus placebo.
LS: least squares; SE: standard error.
Adapted from Ebersbach G et al.19
Moreover, in the opicapone group, dyskinesia was reported almost half as frequently in RMF versus LMF patients (11.8% versus 23.5%), despite similar reductions in OFF-time;19 this might be due to longer disease duration and higher daily levodopa dose in the LMF group.
Opicapone as first-line adjunctive therapy in patients with end-of-dose motor fluctuations
A post hoc analysis evaluating opicapone as first add-on in patients with PD with end-of-dose MF treated with levodopa/DDCI only at baseline (i.e., without dopamine agonists or MAO-B inhibitors) was conducted in 127 patients.20 Baseline characteristics in the opicapone (n=68) and placebo (n=59) groups were comparable, with mean levodopa doses 730.3 mg/day and 718.3 mg/day, respectively.20
Opicapone significantly reduced absolute OFF-time by 68.8 minutes (p=0.0161) and increased ON-time by 79.8 minutes (p=0.0049) compared to placebo (Figure 3),20 while the incidence of potentially related treatment-emergent adverse events leading to discontinuation was similar for opicapone 50 mg (n=5, 7.4%) and placebo (n=5, 8.5%).20 The most frequently reported (≥5% of patients) potentially related treatment-emergent adverse event was dyskinesia (opicapone: n=8, 11.8%; placebo: n=1, 1.7%).20
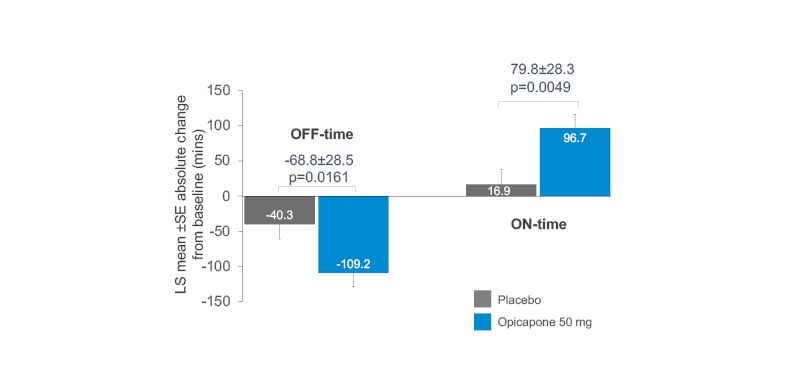
Figure 3: Mean changes in OFF- and ON-time: opicapone as first-line adjunctive therapy versus placebo.
LS: least squares; SE: standard error.
Adapted from Ferreira J et al.20
These data show that opicapone is effective and generally well-tolerated as a first-line adjunctive therapy in levodopa-treated patients with PD and MF.20
Opicapone efficacy in patients with low levodopa doses (300–400 mg) or <4 levodopa intakes
Two further analyses provide evidence of opicapone utility in the treatment of early MF. By combining matching efficacy data for opicapone 50 mg and placebo from the pivotal Phase III studies, different levodopa regimens were evaluated in a subgroup analysis (n=239 patients).21 Improvements in OFF-time were observed for both low-dose and higher-dose levodopa regimens on addition of opicapone 50 mg, with at least a two-fold greater reduction in mean OFF-time versus placebo (Figure 4).21
Figure 4: Mean changes in OFF-time by different levodopa regimen: opicapone versus placebo.
SEM: standard error of the mean.
Adapted from LeWitt PA J et al.21
Another analysis demonstrated a significant improvement in absolute OFF-time from baseline, regardless of whether patients were receiving <4 (-124.5 min; p=0.0397 versus placebo) or ≥4 levodopa intakes (-114.1 min; p=0.0001 versus placebo).22
Reichmann ventured that in the case study described (Table 1), consideration for using opicapone in this patient is backed up by this therapy’s efficacy with low-dose levodopa.21 Opicapone may be useful to keep levodopa doses in the optimised range16 and its once-daily dosing regimen23 might aid adherence, since the more levodopa doses per day, the more adherence inconsistencies.24
Ongoing Opicapone Trials: Supporting Evidence-Based Choices in Treating Motor Fluctuations
Four studies are ongoing to further elucidate the best use of levodopa and opicapone in treating MF and MF-related non-motor symptoms in clinical practice. These trials are being conducted at European sites.
The ADOPTION study is a Phase IV, randomised, prospective, open-label exploratory trial with patients recruited from Germany, Italy, Portugal, Spain, and the UK.25 The aim of this study is to explore the potential of opicapone to optimise levodopa/DDCI as a first-line approach to treat wearing-off (stable treatment plus addition of opicapone 50 mg versus an additional 100 mg levodopa) in 100 adults with signs of wearing-off for <2 years, and treated with 3–4 daily oral levodopa doses up to 600 mg. The primary endpoint is change from baseline in OFF-time at 4 weeks, according to Hauser’s home diary.
The aim of the Phase II, open-label 203 trial26 is to assess the effect of opicapone 50 mg on levodopa pharmacokinetics in different levodopa/carbidopa treatment regimens in patients with end-of-dose MF. Twenty-four patients will receive five intakes of 500/125 mg levodopa/carbidopa dose for 2 weeks. They will then be randomised 1:1 to either four or five intakes of 400/100 mg levodopa/carbidopa plus opicapone 50 mg for 2 weeks. The primary endpoint compares the pharmacokinetics of levodopa at the end of both 2-week treatment periods.
The OCEAN study is a Phase IV, randomised, double-blind, placebo-controlled trial.27 The aim of this study is to evaluate the effect of opicapone 50 mg with levodopa/DDCI in 140 patients with PD with end-of-dose MF and associated pain, recruited from 50 European sites. The primary endpoint is change from baseline in the King’s Parkinson’s Disease Pain Scale (KPPS), domain 3 (fluctuation-related pain) at 24 weeks.
Finally, the OASIS study is a Phase IV, open-label, single-arm pilot trial.28 The aim of this study is to evaluate the impact of opicapone 50 mg as adjunctive therapy to levodopa/DDCI on PD-associated sleep disorders in 30 patients with wearing-off and sleep disorders, at sites in Germany and Portugal. The primary endpoint is change from baseline in Parkinson’s Disease Sleep Scale 2 (PDSS-2) total score at 6 weeks.
Conclusion
Fabrizio Stocchi
In summary, MF are still under-recognised and under-treated in patients with PD. Newer strategies in the form of wearable technologies are now available to help identify MF during a typical patient day. How to integrate and make the most of these technologies together with the ‘new normal’ of virtual clinics in recognising and treating early MF will be key to optimising patient management. While wearable technologies are a welcome addition, mastering gathering a precise patient history with complete description of symptoms, whether by asking key questions or questionnaires, will continue to be paramount for a routine clinical evaluation.
The current evidence supporting opicapone as a potential first-line adjunctive treatment to levodopa/DDCI in patients with PD with early MF is robust. Significant OFF-time reductions have been demonstrated, including for both low-dose and higher-dose levodopa regimens versus placebo, as well as in patients receiving <4 levodopa daily intakes. Data from four further studies, including the potential of opicapone to optimise levodopa/DDCI as a first-line approach to treat wearing-off, in end-of-dose MF-associated pain, and in wearing-off and sleep disorders, aim to consolidate the evidence base for opicapone in routine clinical practice.








