Abstract
Background: Concurrent thyroid cancer and hyperthyroidism is a rare finding. The frequency of this association is very variable. A rare case of papillary thyroid cancer associated with hyperthyroidism is described here.
Case: A 49-year-old male presented to the authors’ outpatient clinic with complaints of a painless left-sided anterior neck swelling that had persisted for the past 8 months. He also reported weight loss for the same duration. The anterolateral swelling was non-tender, asymmetrical, mobile, and rubbery.
Investigations: Biochemical analysis confirmed hyperthyroidism. Ultrasound examination of the neck showed a well-defined, solid, and cystic lesion in the left lobe and isthmus of thyroid gland. The solid portion had few specks of calcification. A radioactive thyroid scan showed increased tracer uptake in the left lobe. Papillary carcinoma of thyroid origin was discovered after fine-needle aspiration of the left anterior cervical lymph node. After preparation, a total thyroidectomy was done. Examination of histopathology confirmed papillary thyroid carcinoma.
Treatment: Following radioactive thyroid ablation, the patient was started on suppressive doses of thyroxine daily.
Conclusion: Although thyroid cancer with hyperthyroidism is a rare finding, it should not be disregarded. To avoid missing this unusual yet uncommon discovery, a detailed history and physical examination should be performed, as well as all required investigations.
Key Points
1. Papillary thyroid cancer coexisting with hyperthyroidism is a rare concurrent finding. Thyroid carcinoma and hyperthyroidism are thought to be related conditions and therefore pose significant diagnostic, therapeutic, and prognostic challenges.2. Concurrent thyroid cancer and hyperthyroidism can present as persistent, painless, left-sided anterior neck swelling with reported weight loss over a period of multiple months.
3. Detailed patient history alongside physical examinations is required to identify unusual findings such as thyroid cancer with hyperthyroidism. All thyroid nodules should be assessed with a high degree of suspicion for malignancy.
Thyroid cancer is the most common malignant endocrine tumour in the world, but it only accounts for 1% of all cancers.1 The most common form of thyroid cancer is papillary thyroid cancer (PTC).2 High-dose external radiation exposure is the most significant risk factor of PTC. Radiation causes genetic lesions that lead to neoplastic transformation.3 The majority of radiation-induced PTC cases have RET/PTC fusions, which are established genetic rearrangements.4
Thyroid hormone development is excessive in patients with hyperthyroidism. Graves’ disease, toxic multinodular goitre, thyroiditis, and exogenous thyroid intake are all common causes of hyperthyroidism.
The coexistence of thyroid cancer and hyperthyroidism is uncommon. A review of literature reports the weighted average of prevalence of malignancy to be 3.1% amongst solitary hyperfunctioning nodules.5 Furthermore, thyroid carcinoma and hyperthyroidism are thought to be related conditions, and, therefore, pose significant diagnostic, therapeutic, and prognostic challenges.5
Unfortunately, due to a lack of population-based data, determining the incidence of thyroid carcinoma in Pakistan is difficult.6
A rare case of PTC and concurrent hyperthyroidism is reported here.
A 49-year-old male with a swelling on the left side of his neck presented to the authors’ outpatient clinic. The swelling was painless and had been gradually increasing in size over 8 months. The patient also reported a significant weight loss of 5–6 kg over the same duration. There were no associated complaints of dyspnoea, dysphagia, hoarseness, heat or cold intolerance, palpitations, changes in urinary or bowel habits, or a past medical history of similar complaints. He was a non-smoker, who denied ever having been exposed to radiation in the head and neck area during his childhood or adolescence. The patient also denied having any family history of thyroid dysfunction or cancer.
The physical examination showed a middle-aged man who was afebrile, had a resting pulse rate of 96 beats per minute, a respiratory rate of 28 breaths per minute, and a blood pressure of 128/70 mmHg. On ophthalmological examination, there was no exophthalmos or lid lag on eye movement. There were no fine tremors and no voice hoarseness.
No thyroid nodule was palpable on neck examination. However, there was a palpable left anterolateral cervical lymph node. This node was asymmetrical, rubbery, mobile, non-tender, and not attached to any overlying or underlying tissue.
Complete blood count was normal. Thyroid function test revealed a thyroid-stimulating hormone (TSH) of 0.010 mIU/L (normal range: 0.4–4.0 mIU/L) and a free T4 of 2.6 ng/dL (normal range: 0.7–1.8 ng/dL). Ultrasound examination of the neck showed a well-defined, cystic, and solid lesion in the left lobe and isthmus of the thyroid gland, measuring 21x11x16 mm. The solid portion measured 9×6 mm, with few specks of calcifications. Sub-centimetre lymph nodes were noted in Levels I, II, IV, and V of the neck bilaterally. The largest lymph node was seen on the left side, which measured 9 mm.
An ultrasound examination of the neck showed a well-defined cystic and solid lesion in the left lobe and isthmus of the thyroid gland (Figure 1).
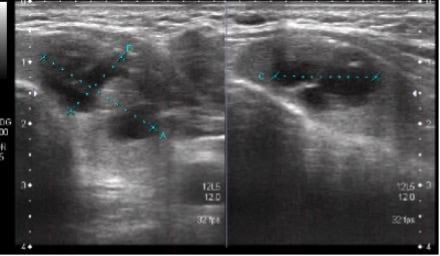
Figure 1: Ultrasound of thyroid.
A radioactive scan was requested to determine thyroid functioning because the thyroid function tests were consistent with hyperthyroidism. These findings were consistent with the presence of a functional nodule. Increased tracer uptake was seen in the left lobe during the scan. The patient was subsequently started on carbimazole and propranolol. The hot nodule was not biopsied because of the very low risk of malignancy. Fine-needle aspiration of the left anterior cervical lymph node was performed to evaluate for sinister pathologies like tuberculosis, lymphoma, or metastasis. The histopathological report revealed metastatic papillary carcinoma of thyroid origin.
Complete thyroidectomy with radical neck dissection was planned. Propranolol 40 mg three times daily and carbimazole 30 mg/day were instituted to prevent a potential thyroid storm during surgery. The patient underwent the surgery without any complications. The presence of multicentric oval nuclei with grooving on histopathological examination confirmed PTC. The specimen sent included the thyroid gland (right lobe, left lobe, and isthmus); Level II, III, and IV lymph nodes on the right; Level II, III, IV, and V on the left; and Level VI of the neck. Bifocal PTC was identified with tumour size of 2.0×1.7×1.0 cm in isthmus and 0.5×0.4×0.3 cm in the left lobe. All lymph nodes were identified as being negative for the disease, except one lymph node in the left Level III measuring 0.1 cm, with no extra nodal extension. Pathological tumour-node-metastases was reported as pT1b N1b.
A haematoxylin and eosin stain of thyroid tissue showed classical PTC, with the cuboidal epithelium exhibiting nuclear clearing, crowding, and intranuclear groove (Figure 2). Calcification is seen at the lower-left corner.
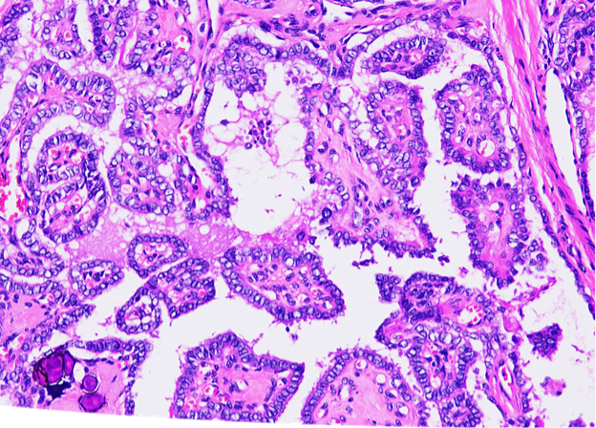
Figure 2: Haematoxylin and eosin stain of thyroid tissue.
One lymph node in left cervical Level III was involved by the tumour. The size of the nodal metastasis was 0.1 cm with no extra nodal extension. All of the other lymph nodes were negative for metastasis.
Post-operative thyroid function test revealed a TSH of 29.4 mIU/mL, thyroglobulin of 0.04 ng/mL (normal range: 20–25 ng/mL), and anti-thyroglobulin antibody of 0.26 IU/ml (normal range: less than 20 IU/mL). The patient received a post-operative dose of radioactive iodine (RAI) 150 mCi. Post-ablative thyroid scan findings were suggestive of functioning residual thyroid tissue in the neck with bilateral cervical lymphadenopathy. To hold TSH below 0.1 mIU/mL, the patient was started on a suppressive dose of thyroxine.
In certain cases, a hyperfunctioning thyroid nodule is thought to rule out thyroid cancer.7 Thyroid cancer and hyperthyroidism may occur as a result of a fortuitous malignancy in the thyroid gland of a patient with clinical hyperthyroidism, or as a rare case of thyroid cancer presenting with hyperthyroidism.8
In patients with hyperthyroidism, the possibility of thyroid malignancy is rare, but should not be disregarded.9 Several studies have reported cases where thyroid malignancy and hyperthyroidism occur together.10-12 Diagnosis relies on histopathological and clinical correlation.
The exact cause of thyroid cancer with coexistent hyperthyroidism is unclear. Thyroid carcinoma can cause hyperthyroidism due to somatic mutations in TSH receptor genes. These mutations increase thyroid levels by activating the cyclic adenosine monophosphate cascade, which causes hormone secretion.9 The combination of mutated TSH receptors and TSH stimulating further growth have been linked to the development of malignancy, but this has not yet been confirmed.13,14
Thyroid cancer caused by an autonomous thyroid nodule needs careful consideration and evaluation. Some factors, however, can aid in determining whether hyperthyroidism is caused by primary hyperfunctioning thyroid carcinoma. These include if there is no improvement in thyrotoxicosis after RAI treatment; if ultrasound findings show hypoechoic stable nodules with microcalcifications; or if there is rapid tumour growth.15 In the authors’ case, the patient did not receive RAI for thyrotoxicosis to monitor any positive or negative treatment outcomes. However, the ultrasonographic findings of a solid lesion with few specks of calcifications were suggestive of underlying thyroid malignancy. Family history of thyroid cancer, a prior history of head and neck irradiation, nodules having microcalcifications, irregular margins and taller-than-wide shape, genetic mutations, tumour size greater than 4 cm, fixation to adjacent structures, and signs of tumour invasion have all been identified as additional risk factors for malignancy.16
Studies point out an increased incidence of follicular thyroid carcinoma (FTC) with concomitant hyperthyroidism. Mirfakhraee et al.5 reported that out of 77 patients, 28 had thyroid carcinoma in a solitary hyperfunctioning nodule. The FTC subtype accounted for the majority of hyperfunctioning thyroid carcinomas.Similarly, Qiu et al.17 found that the FTC subtype was prevalent in 60.5% of functional metastatic thyroid carcinomas, with five cases being hyperfunctioning. Patients with metastatic hyperfunctioning FTC have a poor prognosis compared with patients with PTC. As a result, patients with hyperfunctioning thyroid carcinoma tend to have a high prevalence of FTC, with an especially high prevalence among patients with metastatic disease. However, in this study, the hyperfunctioning thyroid carcinoma is of a papillary subtype with a mild clinical picture of hyperthyroidism.
Two studies, however, reported PTC with hyperthyroidism and extensive disease metastasis. The authors attributed the high level of free thyroid hormones to bone metastasis.18 This explains a relatively innocuous clinical presentation (cervical lymph node involvement with no widespread metastasis) in this case, as the concentration of free thyroxine was within the higher range of the normal level.
The mainstay treatment of thyroid carcinoma with concomitant hyperthyroidism is surgery. This method not only confirms the condition after a pathological examination, but it also removes the cancer and resolves the use of anti-thyroid drugs as a hyperthyroidism treatment, as these are often implemented to prevent a potential thyroid storm. Other options for treatment include RAI fractionation or minimally invasive local ablation.19 In patients with primary hyperfunctioning thyroid carcinoma, RAI is primarily used as a treatment alternative after surgery.20
Thyroid carcinoma and hyperthyroidism coexisting is an uncommon occurrence. Each thyroid nodule should be assessed with a high index of suspicion for malignancy. To prevent ignoring this unusual but important association, thorough medical history and physical examinations should be undertaken, as well as all other necessary investigations.








