Interview Summary
Colorectal cancer (CRC) is the third most common cancer worldwide, and the second leading cause of cancer death. Approximately one in five patients with CRC present with metastatic disease at diagnosis. The BRAF V600E mutation occurs in 8–12% of patients with metastatic colorectal cancer (mCRC), and is characterised by an aggressive clinical course and poor prognosis. This article is based on a webinar discussion in March 2024, between two experts in gastrointestinal cancers, Chiara Cremolini, University of Pisa, Italy; and Julien Taieb, Georges Pompidou European Hospital, Université Paris-Cité, France, both of whom have a wealth of experience and expertise in the clinical management of CRC. The experts described the most important recent advances in the treatment of BRAF V600E-mutated mCRC, including data presented at the European Society for Medical Oncology (ESMO) Congress in October 2023, and the American Society of Clinical Oncology (ASCO) Gastrointestinal (GI) Cancers Symposium in January 2024. Cremolini and Taieb gave valuable insights into topics such as the aggressive nature of BRAF V600E-mutated mCRC, and how this impacts choice of treatment, patient outcomes, and quality of life, as well as the importance of early testing and monitoring. The experts also discussed how the BRAF V600E mutation impacts treatment response and outcomes in patients with microsatellite unstable (microsatellite instability [MSI]) versus microsatellite stable (MSS) tumours, and recent key clinical trials in BRAF V600E-mutated mCRC. The importance of surgery in the multidisciplinary management of patients with BRAF V600E-mutated mCRC, BRAF as a prognostic marker in resected CRC, and real-world studies in this field were also explored. Finally, Cremolini and Taieb described what the future of the management of patients with BRAF V600E-mutated mCRC might look like, and which advancements in research they would like to see.
Introduction
CRC is the third most common cancer worldwide, and the second leading cause of cancer death.1,2 Approximately one in five patients with CRC present with metastatic disease at diagnosis.3 Evaluating biomarkers in the mCRC setting is an important component of clinical practice to guide clinical management and treatment decisions.4-8 Tumour tissue analysis for DNA mismatch repair/microsatellite status and, at a minimum, KRAS, NRAS, and BRAF mutational status is recommended at diagnosis.4 Samples for molecular testing can be obtained by surgical removal of the primary tumour, biopsy of a metastatic lesion, or during colonoscopy.5 Next-generation sequencing has revolutionised the speed and throughput of cataloguing CRC-related genomic alterations,9 and is a useful tool for this important upfront testing. Early molecular analysis is essential to direct treatment, and is important for universal screening for Lynch syndrome.10,11
BRAF V600E mutation, the most common BRAF proto-oncogene mutation,12 is present in 8–12% of patients with mCRC,5,13 and is a well-known negative prognostic factor in non-metastatic,14-17 as well as metastatic,18-20 disease in patients with MSS. This mutation confers an aggressive clinical course,5,13 and is associated with reduced response to some chemotherapies.20 A total of 15% of non-metastatic, and 5% of metastatic CRCs are mismatch repair deficient (dMMR)/MSI, which has prognostic and predictive implications.21 In both non-metastatic and metastatic settings, treatment with immune checkpoint inhibitors (ICI) has shown a remarkable effectiveness in the context of dMMR/MSI status.21 Patients with both BRAF V600E mutation and dMMR/MSI in the metastatic setting had a poor prognosis22 compared to patients with BRAF wild-type before the era of ICIs; however, this prognostic impact of BRAF status in patients with MSI remains controversial when these patients are treated with ICIs. In patients with MSS, median survival in those with BRAF-mutated mCRC can be two- to threefold shorter than that of patients with wild-type tumours (approximately 1 year versus 2–3 years).23-29 Patients with BRAF-mutant tumours often have poor performance status at diagnosis;30,31 therefore, starting a first-line systemic regimen can be difficult, and many patients do not commence therapy.30 A specific therapeutic approach is needed for this aggressive disease, to improve patient outcomes.
Important Advances in the Treatment of Colorectal Cancer in the Last 2 Years
According to Taieb, important advances in the treatment of CRC in the last 2 years include the clinical application of circulating tumour DNA,32,33 and the development of new digital pathology tools to help drive treatment in the non-metastatic setting.34-38 The introduction of new targeted therapies for specific molecular subgroups in the metastatic setting is a positive change that provides additional therapeutic options for patients with CRC; however, the treatment landscape is becoming increasingly complex.39-42
Cremolini stated that the use of immunotherapy for the treatment of mCRC has been a key advancement in patients with dMMR/MSI tumours, in whom immunotherapy dramatically changes prognosis.21,43,44 Targeted options for other molecular subgroups are available, or will be accessible in the near future; however, these treatments have so far not had such a marked impact on prognosis as that seen in patients with dMMR/MSI tumours.43
Importance of Early Testing and Monitoring for Colorectal Cancer
Cremolini emphasised that BRAF mutational status should be determined at the earliest opportunity in the management of patients with mCRC, to guide treatment, and to ensure clinicians do not miss the opportunity to achieve disease control of this highly aggressive, and rapidly progressive, cancer. Knowledge of BRAF mutational status is important in first-line to exclude the use of epidermal growth factor receptor (EGFR) inhibitors,45 which are not particularly effective in patients with BRAF mutation.45,46 Patients with BRAF V600E-mutated CRC should be closely and regularly monitored, to ensure disease progression is identified immediately, and to enable clinicians to offer new lines of therapy as soon as progression is observed.
Taieb reiterated that molecular testing upfront is vital to ensure that patients are treated optimally. In some centres, metastatic disease progression is monitored using CT scan and biomarker testing every 3 months; however, surveillance every 2 months is recommended for patients with BRAF V600E mutation, to identify any rapid progression, and to enable clinicians to offer complementary treatment in second-line.
Impact of BRAF V600E Mutation on Treatment Response and Outcome
Taieb specified that there is an overlap between MSI and BRAF V600E; therefore, it is important to test for both molecular features in patients with mCRC. Most patients with MSI who also have BRAF V600E mutation have sporadic MSI, rather than an inherited MSI, i.e., Lynch syndrome.47,48
The BRAF V600E mutation is a clear marker of poor prognosis in patients with MSS tumours.16,49,50 Individuals with mismatch repair proficient (pMMR)/MSS and BRAF V600E-mutated disease are refractory to immunotherapy,51 whereas those with dMMR/MSI are sensitive to immunotherapy, hence the critical need for upfront testing of microsatellite status. The impact of BRAF V600E mutation in patients with MSI is still debated.52,53 The introduction of immunotherapy has transformed outcomes for patients who have MSI tumours;48,54 however, the BRAF V600E mutation is not predictive of outcomes among patients with MSI mCRC treated with ICIs.48
In line with this, Cremolini pointed out that the KEYNOTE-17755,56 registration trial of pembrolizumab versus standard of care treatment showed that there is no interaction between BRAF mutational status and treatment effect in patients with dMMR/MSI disease.56 Therefore, Cremolini generally prescribes upfront ICIs for all patients with MSI mCRC, regardless of BRAF mutational status.
ESMO Guidelines for BRAF V600E-Mutated Metastatic Colorectal Cancer
ESMO guidelines recommend doublet chemotherapy (5-fluorouracil, leucovorin, irinotecan [FOLFIRI], or 5-fluorouracil, leucovorin, oxaliplatin [FOLFOX]), with or without bevacizumab, in first-line for patients with BRAF-mutated mCRC and MSS tumours (Figure 1).20 In selected cases, when downstaging is the objective, or in right-sided colon cancer with BRAF V600E mutations, a triplet chemotherapy (5-fluorouracil, leucovorin, oxaliplatin, and irinotecan [FOLFOXIRI]), with or without bevacizumab, should be considered, but doublet plus bevacizumab could provide similar outcomes.20
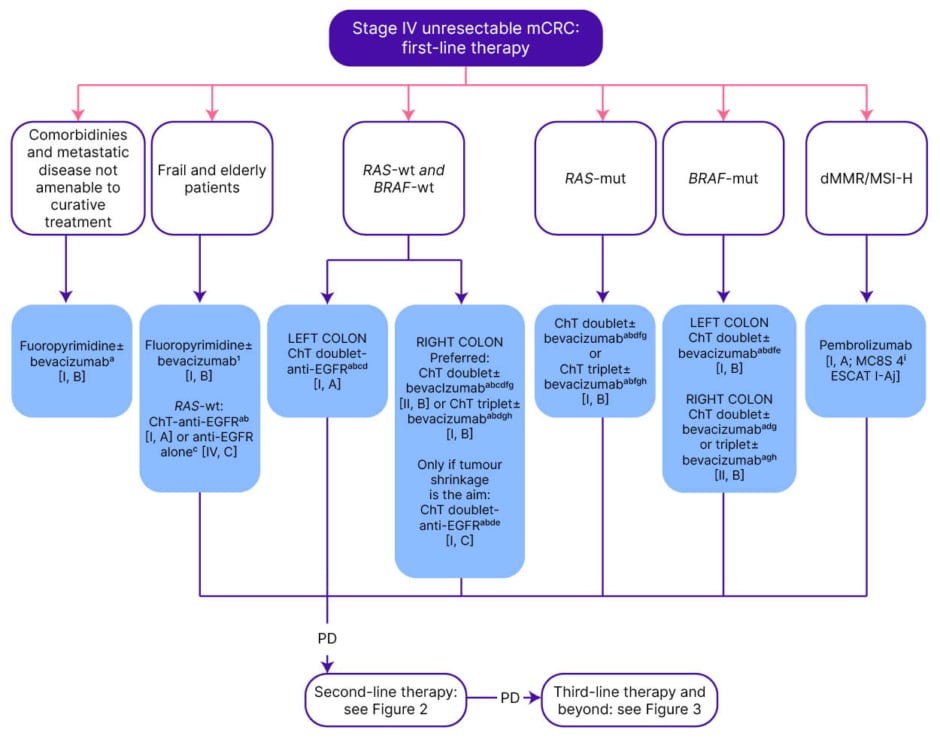
Figure 1: ESMO guidelines: management of Stage IV unresectable metastatic colorectal cancer in the first-line.20
Purple: general categories or stratification; blue: systemic anticancer therapy; white: other aspects of management.
aIn patients presenting with cardiotoxicity and/or hand-foot syndrome on 5-FU or capecitabine-based ChT, S-1 may be used as an alternative [III, B].
bAdditional details on treatments and drug combinations can be found under the section ‘Management of advanced and metastatic disease without potential conversion’ (subsections ‘First-line treatment’ and ‘Second-line treatment’).
cIn frail or elderly patients unable to tolerate ChT, whose tumours are left-sided and RAS-wt.
dFOLFIRI–cetuximab ESMO-MCBS v1.1 score: 4; FOLFOX4–panitumumab ESMO-MCBS v1.1 score: 4; mFOLFOX6–panitumumab ESMO-MCBS v1.1 score: 3.
i,eFOLFOX4–panitumumab ESMO-MCBS v1.1 score: 4; modified FOLFOX6–panitumumab ESMO-MCBS v1.1 score: 3; for FOLFIRI–cetuximab ESMO-MCBS v1.1 score: 4.
i,fIn a very selected population. gCAPOX– or FOLFOX4–bevacizumab ESMO-MCBS v1.1 score: 1.
i,hA triplet with FOLFOXIRI plus bevacizumab is an option for selected patients with good PS and without comorbidities [I, B; ESMO-MCBS v1.1 score: 2].
iiESMO-MCBS v1.1 was used to calculate scores for therapies/indications approved by the EMA or FDA. The scores have been calculated by the ESMO-MCBS Working Group and validated by the ESMO Guidelines Committee.
jESCAT scores apply to genomic alterations only. These scores have been defined by the guideline authors and validated by the ESMO Translational Research and Precision Medicine Working Group.
5-FU: 5-fluorouracil; ChT: chemotherapy; dMMR: deficient mismatch repair; EGFR: epidermal growth factor receptor; EMA: European Medicines Agenc; ESCAT: ESMO Scale for Clinical Actionability of Molecular Targets; ESMO: European Society for Medical Oncology; FDA: U.S. Food and Drug Administration; FOLFIRI: leucovorin–5-fluorouracil–irinotecan; FOLFOX: leucovorin–5-fluorouracil–oxaliplatin; FOLFOXIRI: leucovorin–5-fluorouracil–oxaliplatin–irinotecan; MCBS: ESMO-Magnitude of Clinical Benefit Scale; mCRC: metastatic colorectal cancer; MSI-H: microsatellite instability-high; mut: mutant; PD: progressive disease; PS: performance status; S-1: tegafur–gimeracil–oteracil; wt: wild-type.
Combination therapy with encorafenib, a BRAF inhibitor, and cetuximab, an EGFR inhibitor, has become the standard of care for patients with pretreated BRAF V600E-mutated mCRC, following positive results in the BEACON CRC57,58 trial.59 This combination received approval by the U.S. Food and Drug Administration (FDA) on 8th April 2020,60 and in the European Union (EU) on 5th June 2020.61 ESMO guidelines recommend encorafenib plus cetuximab in second-line (Figure 2), and third-line (Figure 3), for patients with unresectable mCRC.20
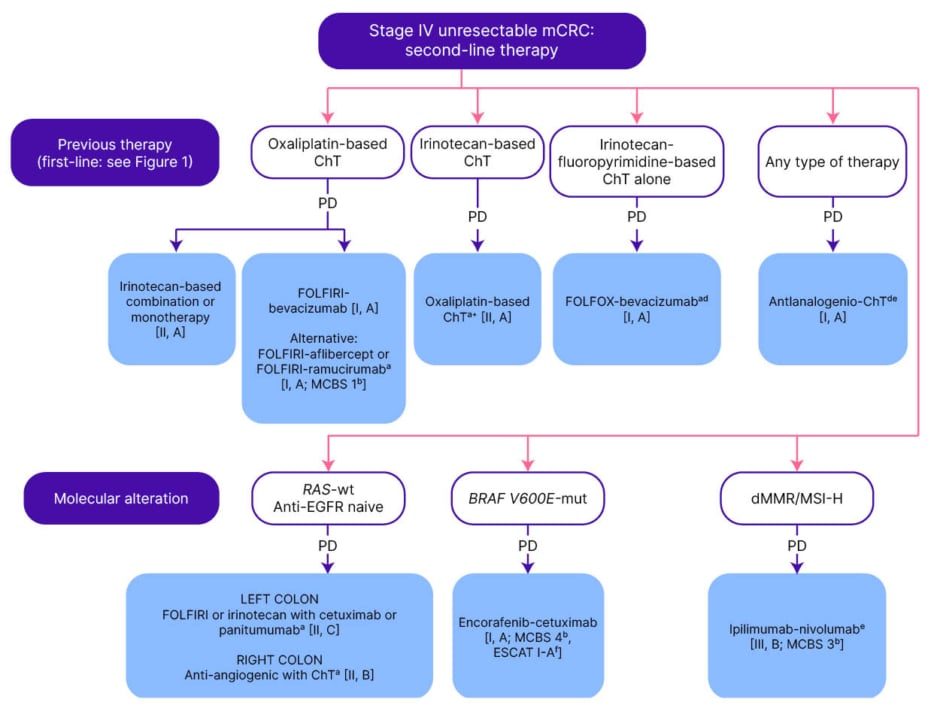
Figure 2: ESMO guidelines: management of Stage IV unresectable metastatic colorectal cancer in the second-line.20
Purple: general categories or stratification; blue: systemic anticancer therapy; white: other aspects of management.
aIn patients presenting with cardiotoxicity and/or hand-foot syndrome on 5-FU or capecitabine-based ChT, S-1 may be used as an alternative [III, B].
bESMO-MCBS v1.1 was used to calculate scores for therapies/indications approved by the EMA or FDA. The scores have been calculated by the ESMO-MCBS Working Group and validated by the ESMO Guidelines Committee (https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-evaluation-forms).
cFOLFOX or CAPOX, if no contraindications.
dBevacizumab can be combined with ChT doublet (a fluoropyrimidine with oxaliplatin or irinotecan, depending on the first-line ChT backbone delivered) [I, A; ESMO-MCBS v1.1 score: 1].
eWith or without previous first-line treatment with bevacizumab and independently of RAS mutational status and the PTL.
fESCAT scores apply to genomic alterations only. These scores have been defined by the guideline authors and validated by the ESMO Translational Research and Precision Medicine Working Group.
5-FU: fluorouracil; CAPOX: capecitabine–oxaliplatin; ChT: chemotherapy; dMMR: deficient mismatch repair; EMA: European Medicines Agency; ESCAT: ESMO Scale for Clinical Actionability of Molecular Targets; ESMO: European Society for Medical Oncology; FDA: U.S. Food and Drug Administration; FOLFIRI: leucovorin–5-fluorouracil–irinotecan; FOLFOX: leucovorin–5-fluorouracil–oxaliplatin; MCBS: ESMO-Magnitude of Clinical Benefit Scale; mCRC: metastatic colorectal cancer; MSI-H: microsatellite instability-high; mut: mutant; PD: progressive disease; PTL: primary tumour location; S-1: tegafur–gimeracil–oteracil; wt: wild-type.
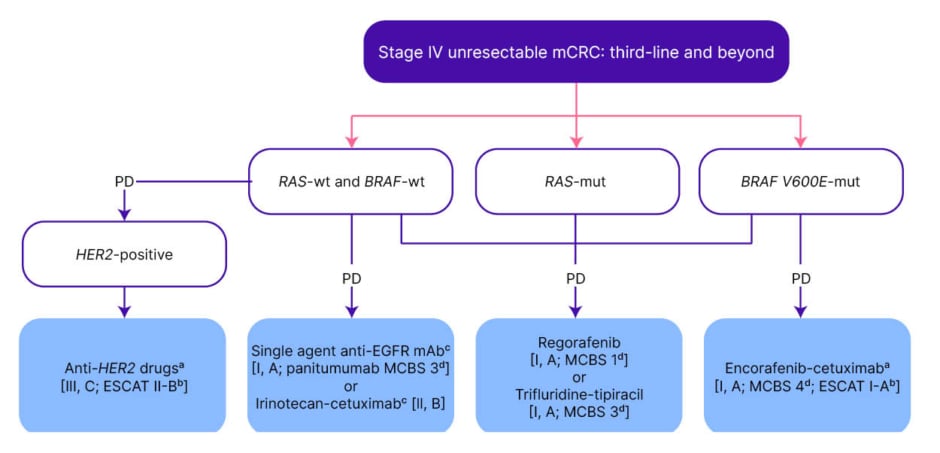
Figure 3: ESMO guidelines: management of Stage IV unresectable metastatic colorectal cancer in the third-line and beyond.20
Purple: general categories or stratification; blue: systemic anticancer therapy; white: other aspects of management.
aFor a summary of recommended anti-HER2 regimens for mCRC see Supplementary Table S6, available at https://doi.org/10.1016/j.annonc.2022.10.003.
bESCAT scores apply to genomic alterations only. These scores have been defined by the guideline authors and validated by the ESMO Translational Research and Precision Medicine Working Group.
cIn patients with RAS-wt not previously treated with anti-EGFR monoclonal antibodies.
dESMO-MCBS v1.1 was used to calculate scores for therapies/indications approved by the EMA or FDA. The scores have been calculated by the ESMO-MCBS Working Group and validated by the ESMO Guidelines Committee.
eTreatment for patients with BRAF-mut if not used in the second line.
EGFR: epidermal growth factor receptor; EMA: European Medicines Agency; ESCAT: ESMO Scale for Clinical Actionability of Molecular Targets; ESMO: European Society for Medical Oncology; FDA: U.S. Food and Drug Administration; HER2: human epidermal growth factor receptor 2; mAb: monoclonal antibody; MCBS: ESMO-Magnitude of Clinical Benefit Scale; mCRC: metastatic colorectal cancer; mut: mutant; PD: progressive disease; wt: wild-type.
Key Clinical Trials in BRAF V600E-Mutated Metastatic Colorectal Cancer
Taieb described recent publications that further evaluated the results of the BEACON CRC57,58 registration trial. These included updated survival analyses, which confirmed the benefit of encorafenib plus cetuximab on overall survival (OS) and progression-free survival (PFS);59 subgroup analyses, which indicated that some patients with multiple markers for poor prognosis may benefit from the addition of binimetinib, a MEK inhibitor, to the combination treatment regimen;59 and an in-depth analysis of safety, which explored timing of onset and resolution of adverse events.62 Taieb explained that the combination of encorafenib with cetuximab is a new therapeutic option for gastrointestinal oncologists; therefore, clinicians need to learn to manage side effects that are outside their usual scope, such as arthralgia and myalgia.62
Cremolini recommended that clinicians should familiarise themselves with the safety data62 on encorafenib plus cetuximab from the BEACON CRC57,58 trial, to enable them to understand the potential time of onset and duration of side effects, and which specific patient subgroups are more at risk of developing adverse events.
ANCHOR CRC63 was a single-arm, Phase II study of encorafenib plus binimetinib and cetuximab in previously untreated BRAF V600E-mutant mCRC. Taieb noted that the results showed promising responses for these targeted agents, without chemotherapy, in the first-line setting, and a manageable safety profile.63
In FIRE-4.5,64 triplet chemotherapy plus bevacizumab versus triplet chemotherapy plus cetuximab showed similar results, with longer PFS in the bevacizumab arm.64 Taieb stated that these results confirm that EGFR inhibitors, such as cetuximab, should not be used in the first-line, but should be administered in the second-line setting.
Another trial of interest was the Phase Ib/II trial, EVICT,65 which combined BRAF and EGFR inhibition, through vemurafenib and erlotinib, respectively, in BRAF V600E-mutated mCRC, and showed activity in terms of overall response rate.65 Cremolini commented that trials such as EVICT65 show that research in targeted therapy is “going in the right direction.”
Importance of Surgery in the Multidisciplinary Management of Patients with BRAF V600E-Mutated Metastatic Colorectal Cancer
More than 50% of patients with CRC develop liver metastases,66 and peritoneal carcinomatosis67 is common in patients with BRAF mutation.68 Cremolini explained that, historically, patients with BRAF-mutated mCRC were rarely offered surgery with radical intent because of the systemic nature of the disease; however, surgery may improve disease control and long-term outcome,31,69-71 and should be an option for these patients.
Taieb concurred that patients with BRAF mutation and resectable metastatic lesions should not be denied surgical intervention; however, clinicians must ensure that all metastatic lesions are identified and resected, which is particularly challenging in the case of peritoneal carcinomatosis.67 Surgical resection remains the only possible cure for advanced-stage CRC;72,73 however, Taieb cautioned against extreme surgery in patients with BRAF mutation because of the high recurrence rates: more than 75% of patients have disease recurrence after surgical resection of the liver for colorectal liver metastases.66,74
BRAF as a Prognostic Marker in Resected Colorectal Cancer
Taieb specified that when analysing the impact of BRAF mutation in patients with resectable, non-metastatic CRC, it is necessary to consider MSI and MSS tumours separately, as these tumours respond differently to treatment.
A pooled analysis of seven clinical trials, comprising 8,460 patients with surgically resected Stage III colon cancer, stratified by MSI/MSS status,15 showed that BRAF V600E mutation is an important prognostic factor for time to recurrence (hazard ratio: 1.58; 95% confidence interval: 1.36–1.83; p<0.0001) and OS (hazard ratio: 2.06; 95% confidence interval: 1.78–2.39; p<0.0001) in MSS disease, but not in MSI disease. Taieb emphasised that these results are clinically meaningful and statistically significant, and may lead to different monitoring and treatment strategies to address the particularly high rates of disease recurrence in patients with BRAF-mutated MSS disease.
According to Cremolini, testing for RAS or BRAF mutational status in patients with Stage II or III CRC is currently not necessary outside clinical trials, as this information does not change the type or duration of adjuvant therapy that the clinician can offer. Knowledge of mutational status may become necessary as research into targeted agents develops in the adjuvant setting.
Real-World Studies in BRAF V600E-Mutated Metastatic Colorectal Cancer
Cremolini highlighted reports of real-world data from Europe75,76 and the USA77 on the use of encorafenib plus cetuximab for patients with pretreated BRAF-mutated mCRC. Real-world data from Italy75 from 133 patients (97 of whom received encorafenib plus cetuximab) showed that the objective response rate, PFS, and safety results overlapped with those from the BEACON CRC57,58 registration trial. The shorter OS observed with encorafenib plus cetuximab in the real-world study (7.2 months)75 compared with BEACON CRC (8.4 months)58 was likely because the patients in the real-world setting were more heavily pretreated than those enrolled in the clinical trial. Cremolini stated that this is an indication to use encorafenib plus cetuximab earlier in the treatment pathway for these patients.
A real-world study of 201 patients in 32 centres (24 of which were in France), presented at ESMO 2023,76 also showed similar efficacy data to that in BEACON CRC,57,58 including a median OS of 9.1 months. A favourable safety profile was also reported, with only 21% of patients experiencing a Grade 3+ adverse event.76
In a real-world study of 125 patients in the USA, presented at ASCO GI 2024,77 77.6% of patients received encorafenib plus cetuximab, and the remaining 22.4% received encorafenib plus an alternative EGFR inhibitor, panitumumab. There were no major issues in terms of treatment duration, compliance, and safety in this study. Cremolini explained that, in cases of infusion reactions, and allergy to cetuximab in patients with BRAF V600E-mutated mCRC, switching to panitumumab is a reasonable option, and the safety and tolerability results for the encorafenib–panitumumab combination from this study were reassuring.
Taieb observed that real-world, post-registration studies generally confirm clinical trial results, even though the patient population tends to be more heavily pretreated, and have lower performance status. Furthermore, the data from real-world studies in BRAF V600E-mutated mCRC across Europe, the USA, and Asia are consistent. The larger patient series in these real-world studies enables the analysis of prognostic factors, which, although not yet leading to changes in practice, are useful to develop new therapeutic strategies based on prognosis. According to Taieb, performance status, the number of previous lines of treatment, and liver and peritoneal metastases are important prognostic factors. Furthermore, real-world evidence may be a useful resource for patients, as it better reflects the likely treatment effects and outcomes than clinical trial data.
Future Prospects and Conclusions
Taieb suggested that the administration of a combination of several drugs upfront may be an effective approach in patients with BRAF V600E-mutated mCRC, because they have such a poor prognosis. BREAKWATER78,79 is an ongoing, global, open-label, multicentre, randomised, Phase III study, with a safety lead-in of first-line encorafenib plus cetuximab, with or without chemotherapy, versus standard-of-care chemotherapy for patients with BRAF V600E-mutated mCRC. Taieb explained that BREAKWATER78,79 will provide important new information about the efficacy, safety, and tolerability of this drug combination administered upfront in these patients. According to Taieb, encorafenib plus cetuximab is well tolerated, with few patients having important toxicities, and the skin rash classically associated with EGFR inhibitors may be slightly reduced with the concomitant use of a BRAF inhibitor in some patients. The effect of adding chemotherapy to this drug combination in terms of tolerability is of interest to clinicians. Taieb would ideally like to see a sequential trial design used to test the efficacy and safety of these treatments, given together or sequentially, in future.
Taieb identified that the BEACON CRC57,58 regimen (encorafenib plus cetuximab) works very well, with tumour shrinkage observed in many patients, although not always reaching the 30% cut-off for a partial response, according to the Response Evaluation Criteria in Solid Tumors (RECIST);80 however, the improvement is short-lasting.13 Treatment strategies, including new drugs or novel drug combinations, are needed to overcome this rapidly developing resistance, so that treatments remain effective for longer.81
Cremolini noted that there are several interesting ongoing trials in which targeted therapy is combined with ICIs in patients with dMMR or pMMR tumours. For example, SEAMARK22 is an ongoing randomised Phase II study, comparing the efficacy of first-line pembrolizumab plus encorafenib and cetuximab versus pembrolizumab alone in patients with previously untreated dMMR/MSI BRAF V600E-mutated mCRC.
In a Phase I/II single-arm trial,82 combining encorafenib plus cetuximab with nivolumab in patients with pretreated BRAF-mutated and pMMR/MSS mCRC, overall response rate was 50%.83 Based on these data, a Phase II randomised controlled trial83 to assess encorafenib plus cetuximab with or without nivolumab in the same setting is ongoing in the USA, and data are awaited with interest.







