Abstract
Erdheim–Chester disease (ECD) is a disease of non-Langerhans cell histiocyte multisystemic proliferation. The pathogenesis is related to accumulation of histiocytes across the body, leading to multiple organ failure, and thus necessitating an early diagnosis. In all ECD cases, BRAF and RAS mutations are critical. Clinical manifestations affect individuals between the fourth and seventh decades of life. The most common symptoms reported are central nervous system involvement with functional disability, and bone pain with osteosclerosis of long bones. Other reported symptoms are skin involvement with xanthelasma, diabetes insipidus, cardiovascular involvement with pericardial effusion and tamponade, perivascular thickening, and right atrial and atrioventricular grove infiltration, leading to heart failure. Females may develop galactorrhoea due to deposition in the pituitary gland, with or without menstrual irregularities. Only few publications address the cardiac MRI findings of ECD. The authors present a case of cardiac involvement of ECD and associated cardiac MRI findings. The patient presented with multisystemic disease with bone pain, diplopia, cardiac arrythmia, and dyspnoea.
Key Points
1. Erdheim–Chester disease is a disease of non-Langerhans cell histiocytosis and clonal disorder, with BRAF V600E mutation and chronic uncontrolled inflammation. In all ECD cases, BRAF and RAS mutations are critical.2. Clinical manifestations involve the central nervous system, cardiovascular system, bones, skin, and eyes. In cases of cardiovascular involvement, patients present with arrhythmias, valvular heart disease, ischaemia, or heart failure. Some patients are asymptomatic. Patients with renal artery involvement present with hypertension.
3. MRI imaging of the heart and bones is important for diagnosis. Cardiac imaging with contrast-enhanced MRI shows late gadolinium enhancement involving the cardiac wall, cardiac valve, aortic root, and aortic wall. Cardiac MRI volumetric evaluation helps in the diagnosis of heart failure.
CASE REPORT
A 54-year-old female with a history of suspected IgG4-related sclerosing disease, who had been struggling with multisystem symptoms for approximately 4 years, presented with worsening diplopia, fever, bone pain, acute kidney disease, arrythmia, and dyspnoea. Patient blood panel revealed haemoglobin of 10.4 g/dL. Urine analysis and infection panel were normal. Immunological studies of serum IgG4 level were normal (16 mg/dL), and serum β2 microglobulin levels were raised (10.2 mg/L). Positron emission tomography (PET) and CT scans revealed hypermetabolic disease in the right kidney, bilateral femurs, and periaortic and pericardial regions. The patient underwent orbitotomy. Mutational analysis showed a BRAF V600E mutation. Femur and renal biopsies were performed, which diagnosed Erdheim–Chester disease (ECD). The patient also developed recurrent pericardial effusions that further confirmed the authors’ suspicions. Cardiac MRI was performed on the MAGNETOM Sola 1.5T MRI system (Siemens Healthineers, Erlangen, Germany), which revealed hyperintense signal within the atrioventricular groove surrounding the right coronary artery, right atrium walls, interatrial septum, and periadventitial region of the ascending aorta. Cardiac MRI also demonstrated diffuse periatrial and periventricular late gadolinium enhancement with atrioventricular groove soft tissue enhancement, and periaortic thickening (‘coated aorta’). Native T1 mapping images showed increased native T1 values at 1,390 (Figure 1). On functional cardiac MRI measurements, left ventricular ejection fraction was reduced due to reduced global systolic function. No significant valvular abnormality was detected. The cardiac involvement in this case was pericardial effusion, detected on chest CT scan (Figure 2). PET imaging identified diffuse pericardiac and periaortic fluorodeoxyglucose uptake (Figure 3).
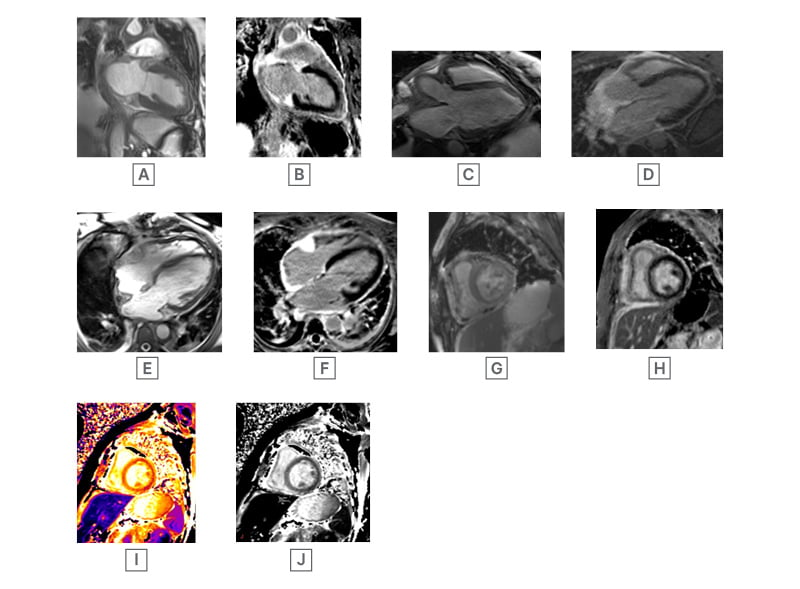
Figure 1: Steady-state free precession cardiac MRI images.
A) SSFP non-contrast two-chamber view, with hyperintense soft tissue around left atrium and ventricle. B) SSFP non-contrast two-chamber view, with post-gadolinium soft tissue enhancement around left atrium, and ventricle with minimal intramyocardial enhancement at apical region. C) SSFP three-chamber view, with periaortic soft tissue. D) SSFP post-contrast three-chamber view, with thickened soft tissue enhancement around aortic root. E) SSFP four-chamber white blood image, with hyperintense soft tissue density mass at right atrioventricular groove, around right atrium and ventricle, and pericardial effusion. F) SSFP four-chamber post-gadolinium, with atrioventricular soft tissue enhancement, and diffuse enhancement in the soft tissue around the right atrium and right ventricle. G) SSFP short axis images, with soft tissue thickening around the right ventricle and inferior wall. H) SSFP short axis postcontrast image, with soft tissue enhancement around the right ventricle and inferior wall. I) and J) Pre-contrast short-axis T1 mapping, with regions of interest drawn in the septum native myocardial T1.
SSFP: steady-state free precession.
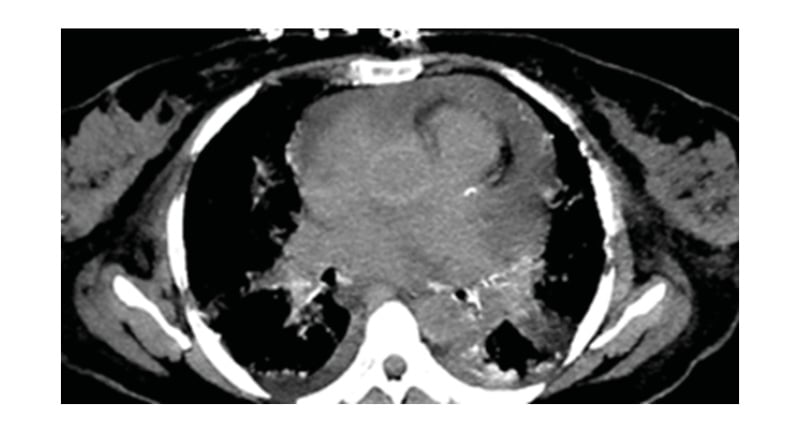
Figure 2: Chest CT (non-contrast study) showing pericardial effusion.
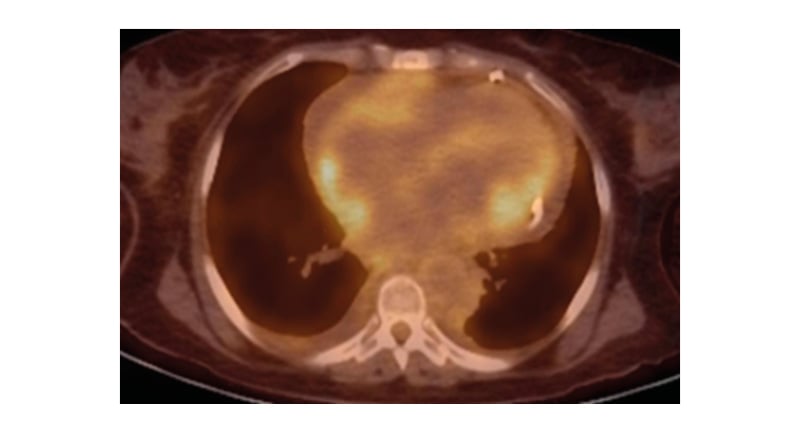
Figure 3: Diffuse fluorodeoxyglucose avid soft tissue around right heart, and focal area of avidity around left ventricle.
DISCUSSION
ECD infiltration usually affects individuals between 40–70 years of age. 1,2 The most common symptom reported is central nervous system involvement, with functional disability and bilateral upper and lower extremity bone pain. More than 50% of patients with ECD present with these symptoms, but some may never experience bone pain. Patients may develop skin manifestations with xanthelasma, hypertension due to renal artery involvement, and diabetes insipidus. Females may develop galactorrhoea due to deposition in the pituitary gland, with or without menstrual irregularities.3,4 The presentation of cardiac involvement by ECD infiltration can be variable, from being asymptomatic to having multiple cardiac symptoms. Patients may present with palpitations due to arrhythmias, valvular heart disease, chest pain due to ischaemia, tamponade, or cardiac failure. Pericardial disease in ECD infiltration can present with pericardial thickening, pericardial effusion, and/or cardiac tamponade. Incidence of cardiac tamponade in patients with ECD infiltration is 13–24%. However, pericardial calcification is rare, and can be seen in up to 4% of patients.5,6 Myocardial infiltration occurs in 25–31% of patients, most commonly affecting the right atrium and right atrioventricular groove, with development of a right atrial pseudotumour.2,3 Another common clinical finding in ECD infiltration is hypertension, secondary to renal artery involvement. ECD causes renal artery infiltration progression to stenosis. In this case report, the patient presented with diplopia and retro-orbital masses. Diplopia was a rare presenting symptom, along with palpitations, dyspnoea, and bone pain.5,7
Pathogenesis reveals a BRAF V600E mutation in most patients, leading to oncogenic alteration. Patients with ECD infiltration also have an oncologic NRAS Q61R mutation, which highlights mitogen-activated protein kinase signalling. The absence of BRAF V600E mutation does not necessarily rule out ECD. ECD infiltration is a purely pathological diagnosis, that is aided by radiological imaging studies. Tissue biopsies classically show an infiltration of bland appearing histiocytes, characterised by abundant foamy cytoplasm with surrounding fibrosis and inflammation. Touton giant cells are frequently present. Immunohistochemistry of histiocytes shows positive staining for CD68, CD163, Factor XIIIa, and S100 (variable); however, does not show expression of CD1a and langerin. Serum analysis of patients with ECD consists of elevated interferon-α, IL-12, and monocyte chemotactic protein-1, as well as decreased IL-4 and IL-7. The case presented here also showed a BRAF V600E mutation, and histopathology of retroorbital masses, which confirmed the diagnosis of ECD.3,4
Imaging Findings
Cardiac and vascular ECD lesions develop due to periadventitial infiltration of the pericardium, myocardium, and coronary arteries, with incidence of 40%. Guidelines for baseline evaluation of ECD now recommend cardiac MRI in all patients, to identify involvement and delineate the extent of disease. The typical appearance of cardiac MRI is a hyperintense mass on T1-weighted images, and hypointense on T2-weighted images, with heterogeneous enhancement following gadolinium administration. Periarterial infiltration of the coronary arteries has been described in up to one-third of patients with ECD, most commonly affecting the right coronary artery, and may cause vascular stenosis and territorial ischaemia. Valvular involvement in ECD is also reported, with the most common valve involved being the mitral valve. It can present with thickening and stenosis of the valve, and progress to valvular insufficiency.4 Myocardial infiltration in ECD is rare, but can lead to cardiac dysfunction, arrhythmias, and heart failure. Patchy late gadolinium enhancement of the myocardium in patients with ECD may also be visualised in a non-coronary distribution. The myocardial infiltration in ECD involves the right atrium, and presents as an ill-defined, heterogeneous mass with variable signal intensity, and can mimic malignancy. Cardiac MRI, in the authors’ case, showed diffuse infiltration in the right atrial, right ventricle myocardium, and aortic root. On functional cardiac MRI, systolic function was impaired, with reduced ejection fraction.5-8
Other radiological imaging findings can be seen with plain radiographs, showing bilateral and symmetric long bone cortical osteosclerosis. Increased fluorodeoxyglucose uptake can be seen on PET imaging around the middle and ends of the long bones, especially in the femur and tibia, present in 95% of patients with ECD.9
The treatment for cardiac involvement in ECD is challenging, and there is currently no standard of care for cardiac involvement. Treatment options include corticosteroids, immunomodulatory agents, and chemotherapy. In some cases, radiation therapy or surgery may be considered. For pericardial effusion, pericardiocentesis or pericardiectomy may be performed. For valvular involvement, valve replacement or repair may be necessary. The prognosis for ECD is generally poor, with a median survival of around 5 years, and cardiac involvement can significantly affect the survival rate.3,10-12
The differential diagnosis for ECD involves pericardial metastasis (e.g., lung, breast), pericarditis, connective tissue disorders, infections (e.g., tuberculosis, fungal, pyogenic), pericardial or cardiac tumour (e.g., myxoma, lipoma, and angiosarcoma), and Rosai–Dorfman disease.3,4