Meeting Summary
Admissions due to asthma contribute substantially to the burden faced by emergency departments (ED) worldwide, with a considerable impact arising from the high number of readmissions among patients with severe asthma. Repeated ED readmittance not only places considerable demand on healthcare resources, but also increases the humanistic burden on patients through reduced lung function, decreased quality of life, and increased exposure to systemic corticosteroids (SCS) and oral corticosteroids (OCS). In addition, patients are subject to the increased morbidity and mortality risk, and quality of life deficit associated with repeated asthma exacerbations.
Admission to the ED should be seen as an opportunity to break this readmission cycle and prevent further admissions, while offering patient-centric benefits, such as investigation into the underlying causes of disease, and optimisation of care to prevent further exacerbations. Actions that require no additional resource may be taken directly in the ED, including biomarker tests among routine blood tests, or teaching inhaler technique as part of patient education and safety-netting. In addition, patient discharge may be considered as an opportunity for improving guidance implementation and breaking the cycle of readmission. Unlike emergency cardiac care, where >90% of patients are discharged on secondary prevention drugs and 85% of patients are referred to follow-up rehabilitation, guidelines for care following an ED visit for asthma are not always followed. Furthermore, current tools designed to accelerate specialist referral are not always rigorously implemented following an ED visit, meaning that follow-up may be delayed. Finally, further efforts should be made to identify high-risk patients in the community earlier in the disease pathway, allowing timely intervention before further lung function impairment, or the onset of adverse events due to OCS over-exposure.
This article summarises an AstraZeneca-sponsored symposium delivered on 12th September 2023, as part of the European Respiratory Society (ERS) International Congress in Milan, Italy. The faculty, consisting of David Price, Head of the Observational and Pragmatic Research Institute, Singapore; Mona Al-Ahmad, Consultant Allergist and Clinical Immunologist at the Ministry of Health in Kuwait; and Mohit Bhutani, Professor of Medicine at the University of Alberta, Edmonton, Canada, each gave a brief presentation on proactive strategies to improve long-term outcomes in acute respiratory care. During panel discussions following each presentation, Anne Marie Marley, Respiratory Nurse Consultant from Belfast Health and Social Care Trust, UK, provided examples of implementing transition of care by bridging hospital and community care settings.
Introduction
Approximately 262 million people have asthma globally, with around 1,000 asthma-related deaths every day.1 In Europe alone, the total direct cost associated with asthma, including drugs, inpatient care, and outpatient (including primary) care, is estimated to be 19.5 billion EUR (at 2011 values).2
The burden of unplanned care for chronic conditions tends to fall disproportionately on EDs, where frequent attendances can turn the ED into a critical pressure point,3-5 particularly during the winter months.6 Asthma is a major contributor to the total number of global ED visits every year,7-9 with 24% of patients (N=8,000) with asthma in 11 European countries having visited an ED in the previous 12 months.10
A disproportionate demand for emergency care is seen among the subset of patients with high-risk asthma, as these patients tend to return to the ED multiple times.11 In a longitudinal retrospective cohort study in Canada, the average number of ED visits for asthma exacerbations between April 2015–March 2020 was highest among patients with Global Initiative for Asthma (GINA) Step 5 asthma (0.5 visits/patient/year) compared with a similar rate of 0.3 visits/patient/year among GINA Steps 1–4.11 By 90 days, the readmission rate for patients with GINA Step 5 asthma was approximately 50% (Figure 1).11 The same study also showed that ED return within 7 days was highest among GINA Step 5 (28.4% of patients), compared with approximately 15% across all GINA stages, while approximately 25% of all ED visits had an outpatient visit in the preceding 30 days (from 21.4% to 35.6% among GINA 2 and GINA 5 patients, respectively).11 It should, therefore, be a matter of priority to break the cycle of ongoing readmissions in order to reduce the burden on acute respiratory care, and proactively improve long-term outcomes in severe asthma.
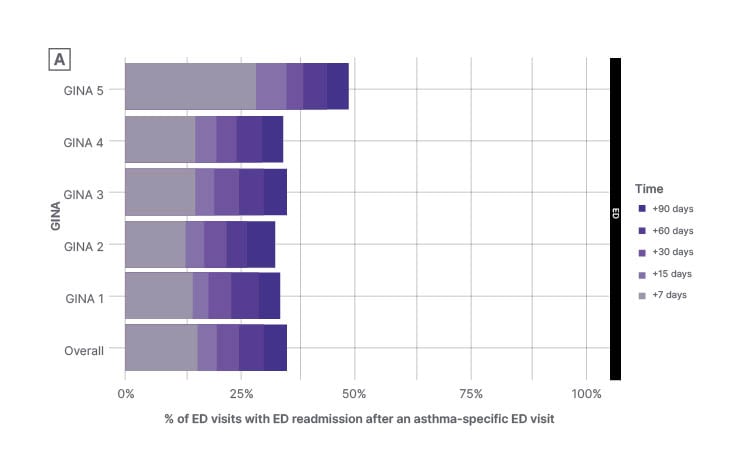
Figure 1: Percentage of emergency department visits with emergency department readmissions after an asthma-specific emergency department visit* (by baseline asthma severity).
*All asthma-specific ED visits are included in this analysis (i.e., a patient may contribute to multiple visits over their entire follow-up).
Longitudinal retrospective cohort study (April 2015–March 2020).
Figure adapted with permission from Mayers et al.11
ED: emergency department; GINA: Global Initiative for Asthma.
How Can We Address the Burden of Unplanned Hospitalisations?
The ongoing burden of unplanned hospitalisations on healthcare resources remains a key healthcare challenge within the current era of precision medicine. Exacerbations can irreversibly decrease lung function in asthma, including a decline in forced expiratory volume in 1 second over the course of several years,12 while also increasing the risk of death.13 A cohort study of more than half a million patients with asthma in five European countries reported all-cause mortality in the week following a severe asthma exacerbation of between 14.1–59.9/1,000 person-years.13
High rates of exacerbations lead to increased ED admissions, and a corresponding higher exposure to OCS and SCS.14 While OCS therapy can be lifesaving in the acute setting, and is recommended in the GINA guidelines for the treatment of exacerbations in the ED,15 increased cumulative exposure to SCS/OCS through frequent ED readmissions increases morbidity among patients with asthma.16 It is concerning that the proportion of patients receiving OCS and SCS upon discharge from the ED is currently increasing.14
The known impact of cumulative OCS doses on adverse events and mortality provides a strong rationale for introducing strategies to reduce ED readmissions. However, despite the evidence of their benefit, secondary prevention measures are frequently not adequately implemented prior to, or immediately after, ED discharge.7,17
Although the ED is a resource-constrained setting focused on acute care, strategies can be integrated into ED management plans to support decision-making, early identification, and specialist input for patients at risk of exacerbations. There are a number of examples of different pathways, biomarkers, and tools that are available to improve patient outcomes (Table 1). The OCS Charter attempts to help clinicians reduce reliance on OCS by recommending education on specialist assessment, new treatment options, and more appropriate OCS prescribing.18 The Oxford Asthma Attack Risk (ORACLE) scale is a tool that uses biomarker variables, such as fractional exhaled nitric oxide and blood eosinophils, to assess risk of future exacerbations, and allows treatment to be targeted appropriately.19,20 ReferID was developed and funded by AstraZeneca (Cambridge, UK) as a web-based tool that healthcare professionals can use to help identify patients with uncontrolled suspected severe asthma, and thus determine whether referral to an asthma specialist is appropriate.21
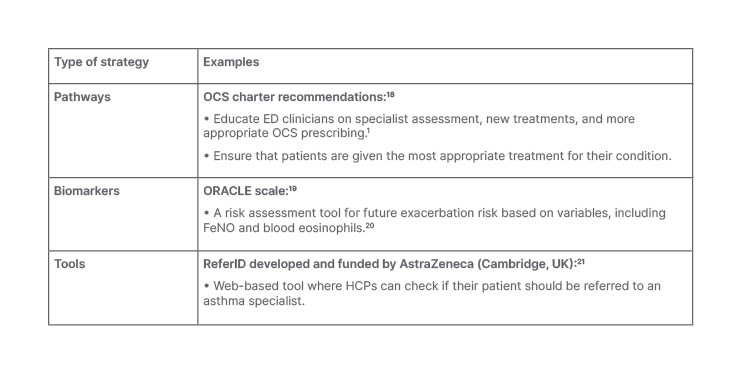
Table 1: Potential strategies and interventions in the acute respiratory setting prior to patient discharge in high-risk asthma.
ED: emergency department; FeNO: fractional exhaled nitric oxide; HCP: healthcare professional; OCS: oral corticosteroid; ORACLE: Oxford Asthma Attack Risk.
Given the impact that inappropriate care, both in the ED and on discharge, can have on patient morbidity and mortality, it is essential that appropriate guideline-recommended treatment is initiated at both timepoints. This includes taking steps to facilitate timely follow-up and early referral, and specialist input for appropriate patients to confirm the diagnosis and determine management and treatment approaches, which may include OCS sparing strategies.15 In a prospective cohort study among patients with severe asthma and high OCS exposure, initiation of biologics was associated with a reduction of 0.09 in the risk of asthma-related ED visits, which is equivalent to a 65.0% reduction in comparison with patients not initiated on biologics (p=0.003; Figure 2).22 Initiation of biologic therapy following appropriate specialist referral may also have cost-saving potential, considering that the mean direct cost of treating a hospitalisation for a severe exacerbation has recently been estimated at 4,997 EUR per exacerbation.22
In order to improve outcomes for all patients, and to reduce the risk of readmission, the ultimate goal should be to initiate multidisciplinary team support for every patient who visits the acute respiratory setting with an exacerbation.
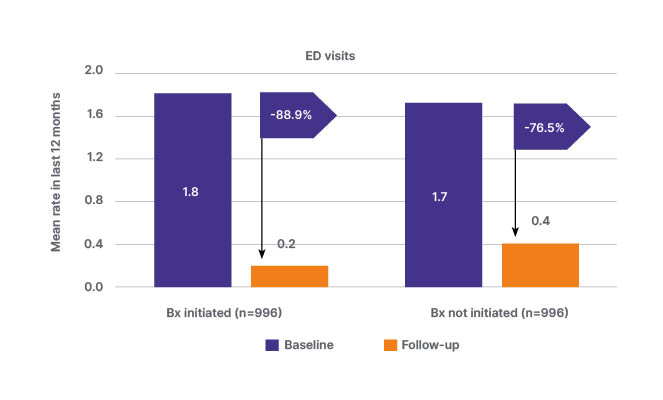
Figure 2: Change from baseline in asthma-related emergency department visits among patients who initiated and did not initiate biologic therapy (prospective cohort study; January 2015–February 2021).22
Propensity score-matched, prospective cohort study using data from the International Severe Asthma Registry (January 2015–February 2021) among patients with severe asthma and high oral corticosteroid exposure. Biologic initiators were matched 1:1 with non-initiators.
Figure adapted with permission from Chen et al.22
Bx: biologic therapy; ED: emergency department.
The first panel discussion highlighted the need to improve the handover of care at the transition between the ED and the community. Marley noted that a large number of patients are lost to follow-up on discharge, and that respiratory experts should be hyperaware at the transitions between care sectors to support risk-stratified approaches to managing asthma patients, rather than all ED visits being considered the same. Bhutani commented that while emergency care focuses on immediate resolution of problems, the long-term consequences of the asthma remain unaddressed, further increasing the importance of early identification of high-risk patients in this setting.
The discussion also considered the importance of communication at all stages of the care pathway. It was noted that patients may not be aware of the severity of their condition before they are admitted, and it is not clear whose responsibility it is to inform them. It is also essential that accurate communication is provided back to primary care when a person has been to the ED. Marley considered that respiratory specialists have a responsibility to work with these teams to ensure appropriate management is implemented following an ED admission.
How Can We Integrate Learnings From Cardiology to Improve Hospital Discharge and Prevent Readmission?
When a patient comes into the ED with a myocardial infarction, discharge guidance includes steps to prevent recurrent myocardial infarction. This contrasts with the situation in respiratory medicine, where there is good knowledge of pharmacological and non-pharmacological strategies that can positively impact outcomes, and reduce the chance of another exacerbation; however, these approaches are not currently maximised. In addition, key checks for asthma are poorly implemented post-discharge,23 unlike the post-myocardial infarction environment where >90% of patients are discharged on secondary prevention drugs, and 85% of patients are referred to follow-up rehabilitation.24
Hospital admission should not be considered a failure in asthma management, but rather an opportunity to change care and prevent further hospitalisations. There is plenty of evidence to demonstrate that specialist care can improve outcomes of patients with severe asthma, and that delayed referral is an avoidable factor related to deaths from asthma.25 The UK National Review of Asthma Deaths (NRAD) report by the Royal College of Physicians (RCP), London, UK, states that of all patients who died of asthma between February 2012–January 2013 (N=195), most (57%) were not under specialist supervision during the year prior to death.25 Furthermore, during this period, only 43% were managed in secondary or tertiary care.25 The report also found that 10% of patients had died within 28 days of hospital discharge; only 23% had been given a personal asthma action plan; and 25% were considered to have died because of a lack of knowledge of UK guidelines, as determined by expert panels.
Worldwide studies have demonstrated that specialist assessment can lead to improved asthma control, reduced exacerbations, and lowered maintenance doses of OCS. For example, an analysis of 1,140 patients in the UK Severe Asthma Registry (UKSAR) reported that 1 year after specialist assessment and management, Asthma Control Questionnaire-6 (ACQ-6) score was reduced by a median interquartile range of 0.7 (0.0–1.5), while exacerbations and unscheduled secondary care were reduced by 75% (33–100%) and 100% (67–100%), respectively. In addition, forced expiratory volume in 1 second increased by a median interquartile range of 20 (-200–340) mL, and OCS dose was decreased in 67% of patients.26 The Severe Asthma Patient Charter provides recommendations for triggers for referral to an expert respiratory physician, which are if the patient has used OCS for more than 3 months, has had two or more courses of OCS treatment in the past 12 months, has been hospitalised for asthma in the past 12 months, or has impaired lung function despite optimised standard therapy.27
Many excellent tools already exist to help improve patient outcomes, including several respiratory ED and hospital discharge bundles that are available globally, but may be adapted for local use.28-31 For example, the British Thoracic Society (BTS) Asthma Discharge Care Bundle supports patient discharge from the ED,29 while the Australian Emergency Care Institute (ECI) and Agency for Clinical Innovation (ACI) specifies a care plan for patients being discharged from the ED.30 In addition, the American Academy of Allergy, Asthma & Immunology (AAAAI) sets out detailed requirements for an asthma discharge plan.31
While it can be difficult to implement change, there are resources available to support adoption of discharge bundles in local healthcare systems. Tips for implementing discharge protocols in chronic obstructive pulmonary disease (COPD) in real-world clinical practice have been published,32 which could also be appropriate in asthma.4,33 It is important that respiratory specialists seek opportunities to prevent recurrent exacerbations, and should make efforts to switch the perception of hospitalisation as a failure, instead regarding it as an opportunity to improve future outcomes.
During the second panel discussion, Marley briefly described the care bundles that are in place following an admission for an acute exacerbation of COPD across a number of hospitals in Northern Ireland. She described how they have a well-resourced multidisciplinary team, including respiratory nurse specialists in hospitals and community teams, respiratory physiotherapists, psychologists, physiologists, dieticians, and general practitioners (GP) to champion shared ownership with patients, so that the bundle is incorporated into patient management plans. Marley described the approach taken to try to increase the recognition of COPD and asthma among commissioners and payers, by emphasising the mortality risk of poor respiratory care.
Beyond Acute Care: How Can We Prevent Emergency Department Admissions?
Finally, it is important to look beyond the acute setting, and consider ways to prevent the very first hospital admission or ED attendance. Recurrent exacerbations of asthma place a substantial humanistic burden on patients, through reduced lung function,34 decreased quality of life,35 and increased OCS/SCS use,36 while even occasional short courses of OCS can cause serious adverse effects.16
Asthma exacerbations are associated with a faster decline in lung function, particularly among younger patients.34 An historical cohort study of 109,182 patients with asthma in the Optimum Patient Care Research Database (OPCRD) reported that for each additional exacerbation, an estimated additional -1.34 L/min peak expiratory flow rate was lost per year (p<0.001). The largest effect occurred in patients aged 18–24 and 25–39 years at baseline.34
The recent ARRISA trial, funded by the UK’s National Institute for Health Research (NIHR) Health Technology Assessment Programme, aimed to identify high-risk patients with asthma by analysing anonymised, longitudinal medical records of 118,981 patients with actively treated asthma (aged 12–80 years) from UK databases.37,38 Interim 3-year data revealed predictors associated with future attacks, including baseline-year markers of attacks (acute OCS courses, emergency visits), more frequent reliever use and healthcare utilisation, worse lung function, current smoking, blood eosinophilia, rhinitis, nasal polyps, eczema, gastroesophageal reflux disease, obesity, older age, and being female.37 ARRISA demonstrates the value of identifying individuals at risk of adverse asthma events, and flagging their electronic health records, to allow primary care staff to deploy processes of care to improve management.38 Staff were also fully trained on the approach to ensure successful implementation.38
An ARRISA-type strategy allows the development of a register of high-risk patients who are given automatic accelerated access to healthcare appointments, provided with self-management plans, and given support for smoking cessation, access to vaccinations, and adherence monitoring. A pilot study showed a trend to reduce admissions, GP visits, steroid use, and cost of service use following the introduction of an at-risk asthma register in a GP setting (n=26).39
In the final discussion session following this presentation, Al-Ahmad commented that the priority now was to implement approaches such as those seen in the ARRISA trial, by educating the relevant stakeholders, providing all the necessary tools on a common standard platform, and co-ordinating the required actions. A clear call to action to the respiratory community was to utilise their leadership and identify high-risk patients earlier, supporting intervention before lung function is impaired, or adverse effects of OCS occur.
Conclusions
The large number of ED readmissions among patients with severe asthma contributes greatly to the demand faced by EDs worldwide, leading to increased exposure to SCS and OCS, and raising the cost and clinical burden of severe asthma. ED visits should be considered an important opportunity to break the cycle of recurrent exacerbations and prevent further admissions, while reducing the impact on patients by limiting lung function decline and mortality risk. Immediate actions that may be taken in the ED to improve secondary prevention include training on inhaler technique, and use of biomarkers to support identification of high-risk patients.
Guidance is available for the management of asthma both during and after an ED visit; however, in many cases this guidance is not being adopted. Respiratory medicine may be able to learn from other therapy areas, such as cardiology, that have reduced ED burden and readmissions by implementing care pathways and discharge bundles, looking beyond an acute/short-term focus to opportunities to treat underlying disease or causes of exacerbations. In addition, it is important to facilitate earlier identification and referral of high-risk patients from the community earlier in the disease course in order to improve patient outcomes and reduce burden.







