Chairpersons: Ased Ali,1,2 Diana Durieux3
Interviewees: Diane Newman,4 Linda Cardozo,5 Emmanuel Chartier-Kastler,6,7 Chris Harding,8,9 Andrei Krassioukov,10 Stefania Musco,11 Angie Rantell,5,12 Todd Linsenmeyer,13-15 Piet Eelen16
1. Medical Affairs, Continence Care, Convatec, Deeside, UK
2. Yorkshire Regional Spinal Injuries Unit, Mid Yorkshire Hospitals, Wakefield, UK
3. Medical Affairs, Continence Care, Convatec, Søborg, Denmark
4. Division of Urology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, USA
5. Department of Urogynaecology, King’s College Hospital NHS Foundation Trust, London, UK
6. Department of Urology, Sorbonne University, Paris, France
7. Urology Department, University Hospitals Pitié-Salpétrière, Paris, France
8. Department of Urology, Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, UK
9. Translational and Clinical Research Institute, Newcastle University, UK
10.International Collaboration on Repair Discoveries (ICORD), Division of Physical Medicine and Rehabilitation, University of British Columbia, Vancouver, Canada
11. Department of Neuro-Urology, Careggi University Hospital, Florence, Italy
12.Brunel University, London, UK
13.Department of Urology, Kessler Institute for Rehabilitation, West Orange, New Jersey, USA
14.Department of Physical Medicine and Rehabilitation, Rutgers New Jersey Medical School, Newark, New Jersey, USA
15.Department of Surgery (Urology), Rutgers New Jersey Medical School, Newark, New Jersey, USA
16.National Multiple Sclerosis Center, Melsbroek, Belgium
Disclosure: Newman has received research funding from Convatec and National Institutes of Health; has been a consultant for Cosm and Urovant; and is Editor for Digital Science Press. Cardozo has been a consultant for Convatec and Merck Sharp & Dohme; and has been on the advisory board for Convatec. Chartier-Kastler has received funding, been a consultant, and/or invited speaker for AbbVie, B. Braun, Boston Scientific, Coloplast, Convatec, Medtronic, TBF Lab, and UroMems. Harding has been a consultant for Mylan Pharmaceuticals and Teleflex Medical; has received speaker honoraria from Allergan, Astellas Pharma, Convatec, and Medtronic; has received fellowship and travel grants from Medtronic; has received research funding from the National Institute for Health Research and The Urological Foundation; and is chair of the European Association of Urology (EAU) Guidelines Panel for Female Lower Urinary Tract Symptoms. Krassioukov has received research funding from the Canadian Institute for Health Research, Canadian Foundation for Innovation and BC Knowledge Development Fund, International Spinal Research Trust, PRAXIS Spinal Cord Institute, Wings for Life Research Foundation, and U.S. Department of Defense; has been a speaker/consultant for Coloplast; and has served on the advisory board for Coloplast, Convatec, and ONWARD. Musco has received funding, been a consultant, and/or speaker for Convatec, Hollister, Coloplast, and Medice (Arzneimittel Pütter). Linsenmeyer has been a consultant and served on the advisory board for Convatec. Rantell has been a consultant and/or speaker for AbbVie, B. Braun, Convatec, Coloplast, Hollister, Consilient Health, and Laborie. Eelen has been a consultant and received presenting fees from Biogen Idec, Convatec, Merck, and Novartis Pharmaceuticals.
Acknowledgements: Medical writing assistance was provided by Hannah Moir, EMJ, London, UK.
Disclaimer: The views and opinions expressed in this article are exclusively those of the expert panel and do not necessarily reflect those of Convatec. All interviewees received honoraria from Convatec for participation in this global advisory board.
Support: The roundtable panel discussion Advisory Board was initiated by Convatec. The publication of this article was funded by Convatec.
Keywords: Catheter, catheter-associated complications (CAC), catheter technology, FeelClean™ Technology, haematuria, intermittent catheterisation (IC), intermittent catheterisation-associated complications (ICAC), microhaematuria, urethral damage, urethral trauma, urinary tract infections (UTI).
Citation: EMJ. 2023;8[3]:38-48. DOI/10.33590/emj/10306793. https://doi.org/10.33590/emj/10306793.
Interview Summary
With a pre-specified aim of improving the standard of care of those living with intermittent catheter use, a roundtable discussion led by a panel of esteemed international experts convened in early 2023. The discussion provided valuable insights and recommendations regarding understanding the challenges associated with intermittent catheter use and catheter-associated complications (CAC).
Key issues centred on the myriad of complications associated with intermittent catheterisation (IC), including urinary tract infections (UTI), discomfort, urethral trauma, haematuria, and their impact on patient-reported outcomes. The heterogeneity of patient groups included in IC research evidence, and discrepancies in current guidelines emerged as key concerns. The panel acknowledged the lack of consensus and clarity surrounding definitions and classification of several complications related to IC and the heterogenous range of reported outcome measures, highlighting the critical need for establishing unified definitions of IC-associated complications (ICAC), and better-defined patient groups in future research, in order to avoid these issues, and produce more definitive research conclusions.
To promote clarity and consistency in terminology and clinical practice, the roundtable discussion proposed an overarching consensus definition for catheter-related complications of IC and associated endpoints, referring to these as “events that disrupt catheterisation.”
The panel also considered the potential of education and innovative catheter technology as an effective means to address these common issues. Recognising the importance of education, the experts highlighted the need for new definitions and descriptions to improve clarity and consistency in clinical practice, and more research involving the array of complications associated with intermittent catheter use. Furthermore, the discussion shed light on advancements in catheter technology, exploring the potential contributions of emerging innovations, such as next-generation catheter technology like FeelClean™ Technology (Convatec, Paddington, London, UK), in minimising complications and enhancing patient outcomes.
INTRODUCTION
Those living with catheters often face clinical challenges that impact overall health and wellbeing, and report having to “suffer in silence” due to a perceived lack of understanding and education.
Catheterisation is used for various conditions, such as urinary retention; postsurgical bladder management; trauma or urinary tract injury;1,2 neurogenic lower urinary tract dysfunction, such as spinal cord injuries (SCI);3,4 stroke; or multiple sclerosis.5 However, catheter use is associated with complications and risks, including UTIs,6,7 pain,8 haematuria,9 urethral trauma,10 autonomic dysreflexia in SCI,11 psychological trauma, and impaired quality of life.12 These complications may arise from the use of various urethral catheter types, such as indwelling catheters (including urethral and suprapubic), and intermittent catheters.1,3,8,9,13
As a member of the expert panel, Diane Newman, Adjunct Professor of Urology in Surgery at the University of Pennsylvania, Philadelphia, USA, highlighted the growing number of individuals, “living longer and maintaining independence with devices such as urinary catheters.” Newman noted that clear or defined recommendations for healthcare professionals (HCP) on catheter use in international guidelines, such as those issued, for example, by the European Association of Urology (EAU),14 European Association of Urology Nurses (EAUN),15 Urology Nurses of Canada (UNC), Infection Prevention and Control Canada (IPAC),16 and the UK National Institute for Health and Care Excellence (NICE),17 are lacking. Such guidelines should aid HCPs in the selection of the most appropriate catheters based on patient scenarios or situations, lifestyle, and medical conditions. Linda Cardozo, Professor of Urogynaecology and Consultant Gynaecologist at King’s College Hospital, London, UK, stated that such considerations vary for different populations, including females, children, those with SCI, or those who have experienced bladder overdistension following surgery or childbirth. Emmanuel Chartier-Kastler, Professor and Chief of Urology at Sorbonne University, Paris, France, and Urology Department, University Hospitals Pitié-Salpétrière, Paris, France, also highlighted the need to address the basics of IC, which they stated were not adequately covered in the international guidelines (although, it was noted that local-level guidelines and teaching recommendations may address this more effectively).14,17 In general, there is a lack of national and international guidance, as well as agreement/consensus among existing guidelines on catheters, and the management of associated complications. Therefore, the global advisory board emphasised the need for clear clinical recommendations and guidelines, to assist in selecting the appropriate catheter type based on patient characteristics and medical conditions.
The experts acknowledged the existence of disparities in inter-disciplinary practices and research, which are compounded by time constraints, the need to determine the appropriate catheter type, and financial costs. The importance of employing the best technique and ensuring consistency in practice was also recognised.3,13,18,19 The panel expressed concern regarding the heterogeneity of patient groups included in intermittent catheter studies.20-23 Notably, Cardozo, Newman, and Chris Harding, Professor in Urology at Newcastle University, UK, and Consultant Urological Surgeon at Freeman Hospital, Newcastle-upon-Tyne, UK, highlighted deficiencies in evidence-based research. They identified a lack of differentiation in indications and reasons for catheterisation, frequency of IC, as well as catheter types, or detailed information on the evolution of catheter technology in contemporary published research. Consequently, they identified the necessity for better-defined patient groups in clinical studies, which could mitigate these issues and yield more conclusive findings.20-23 Furthermore, different organisations rely on different sources, highlighting the lack of clear guideline recommendations resulting from a paucity of high-level conclusive evidence upon which recommendations can be based.
The roundtable discussion identified limited detailed baseline information regarding study populations and a lack of aligned outcomes specifically in association with catheter-related complications, noting the paucity of robust evidence and detailed information available for several clinical endpoints.
The panel highlighted the importance of providing guidance and education to effectively address the clinical needs associated with IC, and to establish an international consensus on ICACs.
UNDERSTANDING THE CLINICAL COMPLICATIONS AND CHALLENGES IN INTERMITTENT CATHETERISATION
The roundtable discussion revealed that the majority of research on catheter use is primarily focused on indwelling catheters.1,3,12 There is a limited emphasis on IC and its associated consequences,10,20-22 with most of the existing literature predominantly addressing UTIs as compared to other complications.5,19,23 Andrei Krassioukov, Professor in Physical Medicine and Rehabilitation Medicine, Clinician-Scientist at the University of British Columbia, Vancouver, Canada, identified that most clinical trials related to IC are primarily focused on UTIs as an endpoint, and rarely consider other complications or endpoints. Furthermore, Stefania Musco, Consultant Urologist in Neurourology at Careggi University Hospital, Florence, Italy, raised the importance of differentiating between symptoms and complications associated with indwelling catheters versus IC.
The lack of consensus and clarity regarding the complications, outcome measures, clinical endpoints, and intermittent catheter-related issues in the literature necessitates the establishment of unified definitions and recommendations for addressing ICACs. The panel identified that ICACs can encompass various clinical endpoints, including UTIs; trauma, which may be due to injury (visible lesions, tears, or stricture);5,24,25 or microhaematuria;20,21 as well as pain;9,26-29 discontinuation and compliance issues;13 and events that disrupt the ability to catheterise, or lead to end-stage complications like strictures and bladder stones.13,22 Indwelling catheterisation may also include catheter-related hypospadias, catheter blockage, transurethral leakage, bladder neck issues, autonomic dysreflexia in patients with SCI,11 and peri-catheter leakage.12,29
Key Points
1. The roundtable discussion highlighted the lack of consistency in defining and measuring urethral trauma, as different terms or descriptions are often used interchangeably, or without specific definition. Furthermore, there is an emphasis on UTIs in the existing research, while overlooking the importance of other complications such as pain, urethral trauma, or damage.
2. To better guide HCPs in their decision-making, and to address the lived experiences of those performing catheterisation, the expert panel identified that it was imperative to establish clear consensus definitions for research to encompass a wider spectrum of complications, and the need for better-defined patient groups.
3. The roundtable discussion emphasised the significance of considering UTIs, pain, urethral damage, trauma, and complications such as gross haematuria (also termed visible, and formerly macroscopic) and microhaematuria (also termed non-visible haematuria, and formerly microscopic) in the context of ICACs.
4. The expert panel highlighted the importance of providing guidance and education to effectively address the clinical needs associated with IC.
Challenges in Defining Urethral Trauma, Urethral Damage, and Haematuria in Intermittent Catheterisation
The panel acknowledged the challenges in accurately defining and measuring urethral trauma or damage, as it can encompass visible and non-visible lesions, strictures, tears, or even full rupture and distraction. This lack of clarity poses difficulties in accurately capturing and reporting urethral trauma, and the necessity for a unified definition and identified outcome measures due to inconsistent use of these terms in the research, such as urethral trauma, urethral complications, urethral injuries, or urethral damage, without a clear consensus on the precise definition.21,26,29,30
Moreover, many studies primarily focusing on UTIs may not adequately measure trauma or damage. Urethral trauma is a significant concern associated with catheterisation, with Angie Rantell, Urogynaecology Consultant Nurse at King’s College Hospital, London, UK, and Senior Lecturer at Brunel University, London, UK, identifying that it encompasses both physical and psychological aspects. Chartier-Kastler discussed how such clinical consequences and events can impede or prevent catheterisation, and may include self-inflicted trauma, which can lead to a reduction in patients’ compliance with their intermittent catheter regimen. A study investigating urethral epithelial cells as an indicator of urethral trauma after IC in patients with SCI underscored the need for a precise definition of urethral trauma.30
The roundtable discussion debated whether measures of urethral trauma are sufficiently specific to the location, as haematuria, for instance, may also associate with other causes. Newman raised a valid point regarding the challenges in classification and assessing anatomical differences between males and females. Additionally, it was evident that there was a significant over-representation of males in research studies.20-22 There were questions regarding the relative importance of trauma in the bladder versus the urethra, and how to categorise and identify existing gaps in knowledge. It was noted that damage is a long-term consequence, whereas trauma can be acute. However, Newman pointed out that the full extent of urethral damage and its clinical consequences remains unclear.
Todd Linsenmeyer, Professor in Urology and Physical Medicine and Rehabilitation at Rutgers New Jersey Medical School, and Director of Urology at the Kessler Institute for Rehabilitation, both in Newark, New Jersey, USA, noted that there are a number of classifications of urethral trauma. These are focused on significant blunt and traumatic urethral injuries, often involving traumatic disruptions due to pelvic fractures.24,25,31 Linsenmeyer mentioned that these classifications are not very useful when considering catheter-induced microhaematuria. Linsenmeyer proposed that it may be helpful to develop a microhaematuria classification to better characterise catheter-induced microhaematuria, particularly for research studies. This classification could be based on various degrees of microscopic haematuria (non-visible) up to gross (visible) haematuria. In those undergoing a cystoscopic examination, the actual location of the catheter-induced urethral trauma and any possible long-term urethral trauma sequalae, such as false passage, urethral stricture, and meatal stenosis, could be noted.32
The significance of microhaematuria and its association with ICACs is also less clear. Krassioukov acknowledged the difficulty of assessing patients with urethral trauma, where catheterisation was categorised based on cystoscopic examination on the basis of microhaematuria. Harding added: “The only time cystoscopy is carried out in a useful timeframe relative to urethral trauma, is if they are having a catheter inserted or changed, and the patient reports significant visible haematuria.” Harding also noted that currently, there is no clinical evidence on whether microhaematuria (non-visible) contributes to longer-term CACs, such as urethral stricture, recurrent infection, reduced compliance with intermittent self-catheterisation regimens, or bladder stones. The variability of cystoscopy practices and the lack of clear guidelines for its use in relation to urethral trauma further complicates the matter. Linsenmeyer noted that the new American Urological Association (AUA)/Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU) guideline on neurogenic bladder dysfunction as a clinical principle does not recommend routine cystoscopy surveillance on everyone with a neurogenic bladder.32 Cystoscopy is suggested for those with recurrent infections, suspected anatomical anomalies, haematuria, or false passage.33
The Complexities of Defining Urinary Tract Infections in Intermittent Catheterisation
Compliance with IC is crucial in reducing the risk of UTIs, and associated signs and symptoms. Harding discussed the relationship between urethral barrier function, cell damage, urethral damage, and the development of catheter-associated UTIs (CA-UTI).10 Ensuring compliance with IC is essential for minimising the risk of UTIs. Chartier-Kastler criticised the lack of evidence linking technology, lubrication, and CA-UTIs, stating while there is a theoretical link between UTIs and IC, there is insufficient empirical evidence to support this connection with catheter technology. Studies comparing different catheter types and outcomes have yielded mixed results and have lacked clear definitions of UTI and haematuria.4,10,18,19
Differences in UTI definitions and practices between the USA and Europe were also noted, emphasising the need for a unified approach. Various organisations, including the International Spinal Cord Society (ISCOS), the American Spinal Injury Association (ASIA),34 the EAU,35 the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR),11,23 and the Infectious Diseases Society of America (IDSA),36 acknowledge the need to consider both bacterial presence and symptomatic UTIs. However, the clinical definition of a symptomatic UTI is non-specific, and often relies on the confirmation of an antibiotic prescription.6 Both Harding and Linsenmeyer pointed out the limitations of antibiotic prescription studies in terms of accurately discriminating between whether individuals actually had a UTI, or if there was inappropriate antibiotic use.18 Harding acknowledged the limitations of relying solely on microbiological tests as indicators of UTIs, and stated that recent study outcomes defined a UTI as “presenting with a symptomatic UTI requiring treatment and being prescribed antibiotics,” and reported that urine cultures were negative in up to 50% of cases.37
Linsenmeyer noted the importance of confirmation of a UTI in a person presenting with complaints of a possible UTI. The criteria which have been recommended by a consensus conference and UTI data set are bacteria, pyuria, and the new onset of symptoms.11,34 The panel acknowledged the relatively high prevalence of background symptoms among those living with intermittent catheter use and highlighted that the diagnosis of symptomatic UTIs may be based on symptom exacerbation, which may not be accurate in terms of CA-UTI diagnosis. Other possible UTI symptoms based on the consensus guidelines include fever, autonomic dysreflexia in patients with SCI, increased spasticity, discomfort or pain over the kidney or bladder during micturition, onset and/or increase in incontinence episodes, cloudy urine with increased malodour, malaise, lethargy, or sense of unease.11 Additionally, a positive dipstick test for leukocyte esterase was mentioned.6 However, Krassioukov commented that dipstick urinalysis is more commonly used in Europe compared with the USA.
Rantell acknowledged the challenge in defining UTI symptoms, as there can be overlap between symptoms of a UTI and symptoms of urethral trauma. Harding added that those with neurological lower urinary tract dysfunction may not experience any symptoms due to their neurological deficit, despite having a UTI. Linsenmeyer noted the challenge of using different definitions to classify UTIs, highlighting the difficulty in accurately defining and categorising these infections. This indicates significant differences in community and international practices, which can present challenges in identifying a unified definition of CA-UTIs in IC. However, a unified definition would help to improve understanding and facilitate evidence-based research.
From a patient perspective, challenges associated with IC, including UTIs and discomfort, were highlighted by Cardozo, noting the impact on quality of life. Linsenmeyer noted that bladder overdistension is one of the most common causes of UTI, due to the resulting bladder wall ischaemia.38 Linsenmeyer highlighted the importance of making self-catheterisation more comfortable in order to improve patient compliance, and thereby reduce the incidence of UTIs. Additionally, they highlighted the significance of educating patients on fluid intake, and best catheterisation practices, thereby promoting the role of education in this context.
ADDRESSING THE LIVED EXPERIENCE OF THOSE USING INTERMITTENT CATHETERS
The discussion highlighted the importance of considering the lived experience of IC, with a focus on avoiding UTIs, urinary incontinence, and achieving patient satisfaction. Newman also mentioned that patients report observing visible blood in the catheter, which may be indicative of trauma, or gross haematuria. However, Chartier-Kastler commented that, currently, there is no evidence linking this gross haematuria to clinical consequences. Newman also highlighted the “challenge of the catheter sticking and the adhesiveness of certain coatings,” emphasising the impact this can have on catheter selection, and on compliance with frequency of catheterisation. Newman acknowledged the patient perspective, suggesting that patients are aware of catheters ‘sticking’ particularly during removal, with reports of the patient needing to use force during removal. Patients commonly use phrases such as “can’t pull it out,” or “have to be pulled out,” and express discomfort during catheter removal, indicating that these issues may be more prevalent than reported. The panel discussed the issues of HCPs being unaware that ‘sticking’ can be due to the catheter coating. The roundtable discussion emphasised the importance of HCPs understanding that certain hydrophilic-coated catheters can cause this ‘sticking’ sensation.9,27
Concerns were raised regarding the cellular repair time of urethral damage between ICs. Newman and Piet Eelen, Clinical Nurse Specialist in Multiple Sclerosis at the National Multiple Sclerosis Center, Melsbroek, Belgium, identified that insufficient time for repair before the next catheterisation has unknown consequences. Additionally, Rantell highlighted the need to investigate the acclimatisation of epithelial barrier proteinisation, questioning the extent of damage, the potential for cellular death, and the unknown consequences that may arise as a result. These concerns highlight the need for further investigation in this area.
Rantell suggested that consideration of patient satisfaction and patient-reported outcomes was therefore just as crucial as clinical endpoints in terms of evaluating treatment success. They suggested the focus should extend beyond device-related complications, and consider factors like motivation, lifestyle, and psychological aspects that may impact patient satisfaction. Validated questionnaires and measures, such as patient global impression of improvement,39 and quality of life indicators, such as the IC Difficulty Questionnaire,40 and IC Satisfaction Questionnaire,41,42 should therefore be included as patient-reported outcomes. Incorporating mixed methods research, including qualitative and quantitative measures, and gaining insights from the patients’ lived experiences are crucial for a comprehensive understanding of catheterisation, and the factors contributing to complications. Follow-up studies and reflections on patients’ journeys can provide valuable insights for improving care.
Key Points
1. Patient adherence to catheterisation protocols was recognised as a challenge that needs to be addressed.
2. Success of IC should take into account the patient’s perspective, such as comfort, ease of use, and overall satisfaction.
3. The panel agreed that a successful single catheterisation episode should be characterised by comfort, ease, quickness, and be painless.
UNDERSTANDING INTERMITTENT CATHETERISATION-ASSOCIATED COMPLICATIONS TOWARDS CONSENSUS DEFINITIONS, AND OUTCOME MEASURES
The roundtable discussion acknowledged the interchangeable use of terminology across practices and scientific literature. Cardozo highlighted that this interchangeability can lead to “different interpretations among different audiences,” thus limiting the ability to draw meaningful comparisons between studies.
The roundtable discussion underscored the need for a comprehensive consensus definition for ICACs, that encompasses “events that disrupt catheterisation” (Table 1). Such a definition is crucial to ensuring clarity and consistency in clinical practice, and establishing a core outcome set is crucial for future studies. The roundtable discussion highlighted the importance of considering clinical factors in addition to UTIs, including urethral damage, urethral trauma, and other potential complications, when discussing IC (Table 1).
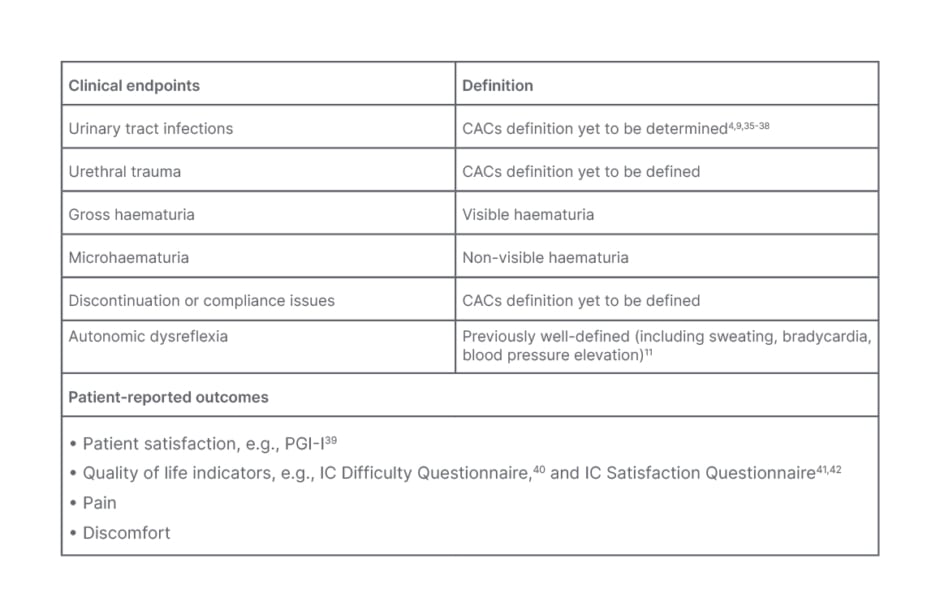
Table 1: Clinical endpoints and patient-reported outcomes of catheter-associated complications in intermittent catheterisation.
CAC: catheter-associated complications; IC: intermittent catheterisation; PGI-I: Patient Global Impression of Improvement.
The panel was unified in their agreement that urethral trauma can lead to stricture, which refers to the narrowing of the urethra as a result of scarring or inflammation. Microscopic haematuria refers to non-visible blood in the urine, while gross haematuria involves visible bleeding that can disrupt a patient’s ability to perform catheterisation, and lead to other CACs such as UTIs. However, what remains to be defined is discontinuation and/or compliance.
Reducing the Knowledge Gap in Catheter-Associated Complications
The discussion also identified the importance of enhancing education and empowering HCPs and patients regarding the risks and consequences of CACs.
Newman shared clinical experience from the USA, noting that nurses often miss opportunities for prescribing IC, as opposed to indwelling catheterisation, due to concerns about patient harm and a lack of procedural training. Rantell acknowledged the challenges in the teaching of catheterisation and managing complications associated with IC. Newman added that nursing education in the USA often lacks comprehensive teaching and practical training around catheterisation. The use of a “checklist” when teaching a patient to self-catheterise can be helpful.43 Krassioukov also identified that adherence among patients was a challenge, with misconceptions regarding bladder infections caused by self-catheterisation, particularly among individuals with SCI. A study conducted in the USA demonstrated the benefits of a nurse-led education and training programme in reducing CA-UTIs.7 Harding said that the study “underlines the importance of having education programmes” to promote proper catheterisation practices and improve patient comfort. Chartier-Kastler stressed the need to educate HCPs on new approaches, training, and appropriate catheter selection based on clinical needs. Harding added the importance of the clinical teams’ initial catheter choice, but also highlighted the need for timely follow-ups to address any issues, to promote adherence to intermittent catheter protocols, and to explore different catheter products if necessary.
The gap in knowledge and competency potentially contributes to the reluctance to perform IC. This may lead to potential patients who would be candidates for IC being inappropriately managed with indwelling catheters. The discussion recognised the need to address this gap by providing adequate education and training, covering topics such as appropriate catheter choice, proper techniques, and ongoing relevant education to equip HCPs with the necessary skills and knowledge.
Advancements in Catheter Technology in Addressing Catheter-Associated Complications
One challenge linking CACs and catheters is their coating. Catheter sticking and the adhesiveness of coatings are significant challenges reported by patients, which can impact catheter choice and cause discomfort.8,9,27 Musco identified that there was no specific mention of the risk of infertility with IC in guidelines, although differences in catheter types, and catheter residues, could be amongst the factors that impact the quality of sperm in patients with spinal cord injury.44 Newman added: “The dilemma in this area is the rapid changes in catheter technology, which continues to evolve.”
The panel discussion shed light on the advancements in catheter technology, such as technology where the hydrophilic properties are integrated and not adhered to the catheter surface as a coating. An example of this is the FeelClean™ Technology (Paddington, London, UK). This next generation technology may contribute to minimising CACs and improving patient outcomes.
Hydrophilic-coated catheters have been noted to become adhesive as they dry, which has been associated with sticking of the catheter, where increased force is required to withdraw the device.45-47 As such, the roundtable discussion proposed that HCPs need to be aware that hydrophilic-coated catheters can cause sticking issues,8,9,27,45-47 emphasising the importance of understanding in order to address patient concerns, and provide appropriate guidance to minimise discomfort and potential CACs. As mentioned by Newman, patients report challenges with catheters sticking during removal. This can impact catheter choice and cause discomfort, which Newman feels may negatively affect patient compliance. However, newer ‘next generation’ catheter technologies, such as those with integrated amphiphilic surfactant (FeelClean™ Technology), do not become sticky as they dry, and there is no coating, so no shedding of residue,45 and may reduce urethral microtrauma.46,47 Linsenmeyer noted that the FeelClean™ Technology is not a typical “uncoated” catheter, but a unique type of hydrophilic catheter. Such advances in catheter care may alleviate common issues such as CACs, including urethral trauma and potentially patient discomfort.45-47
SEEKING A CONSENSUS ON INTERMITTENT CATHETERISATION-ASSOCIATED COMPLICATIONS
The experts agreed on key themes and challenges regarding the use of intermittent catheters, such as defining ICACs as events that disrupt catheterisation, and the expert panel emphasised the need for future research.
The discussion highlighted the current reactive approach to symptoms, and the need to emphasise prevention of CACs and problems in catheter care. Proactive strategies to address complications before they become major issues were deemed necessary.
Measuring outcomes and understanding the implications of ICACs remains a challenge, particularly in relation to outcomes such as microscopic haematuria, and the relationship with complications such as strictures and discontinuation. Long-term epidemiological studies are needed to understand the cumulative effects and complications associated with IC and patient compliance with intermittent catheter regimens. To enable specific recommendations for IC, it is also necessary for research to strive for homogenous patient populations. Additionally, the underrepresentation of females and paediatric populations in research was noted.
In summary, the expert advisory board’s roundtable discussion offered valuable insights for HCPs on ICACs. By establishing unified definitions, improving education initiatives, patient comfort, and embracing technological advancements such as FeelClean™ Technology, significant progress may be made in reducing complications, and enhancing patient care in intermittent catheter management. However, it is important to note that further high-quality research is necessary to confirm the effectiveness of these approaches.







