Abstract
Acute encephalopathy, caused by infectious agents, metabolic or mitochondrial dysfunction, brain tumor, or prolonged exposure to toxic elements, is a significant cause of morbidity and mortality in young children. Lead encephalopathy that occurs due to prolonged lead ingestion or exposure in children is not uncommon, and many such cases have been reported in the past. This report shares the authors’ experience of the missed diagnosis of lead encephalopathy, its red flags, and its relation to pica as a root cause. A 14-month-old boy, resident of Delhi, India, was admitted with vomiting, irritability, and two episodes of abnormal limb movements, with the first symptom starting when he was 11 months old. His case was initially treated as acute disseminated encephalomyelitis, but he showed no improvement. The child was then referred to the Sir Ganga Ram Hospital, New Delhi, India. The boy presented with severe anemia, along with basophilic stippling on the peripheral smear during routine investigations. Suspecting lead poisoning, blood lead levels were checked, and were found to be grossly elevated. The child was then started on chelation therapy. Multiple doses of chelation (dimercaprol and D-penicillamine) were given, and sequential blood lead levels were monitored, which showed a marked decrement. The child was discharged, and is being monitored routinely. Lead poisoning remains a neglected public health issue, especially in children, and delay in identification can cause significant morbidity and mortality. Pediatricians and general practitioners must be aware of this risk, especially in children with iron deficiency anemia exhibiting pica.
Key Points
1. Despite being treatable, lead poisoning and lead encephalopathy are still critical public health concerns, especially among children, primarily due to delayed diagnosis.2. This case report aims to demonstrate how lead encephalopathy can clinically mimic other encephalopathic conditions, like acute disseminated encephalomyelitis (ADEM), potentially causing oversight and delay in diagnosis.
3. Children with symptoms of lead encephalopathy may show clinical similarities to conditions like ADEM or viral encephalitis. Thus, when conventional therapies fail, clinicians should consider lead poisoning as a potential differential diagnosis.
INTRODUCTION
Acute encephalopathy is a significant cause of morbidity and mortality in young children. It can be caused by infectious agents, metabolic or mitochondrial dysfunction, brain tumors, or prolonged exposure to toxic elements like lead, which can result in poisoning, leading to severe consequences, including encephalopathy. Lead is a naturally occurring heavy metal found in the earth’s crust. It can gain entry into the body through various pathways, including lead-based paints, contaminated soil, construction dust, and water.1 The Global Burden of Disease (GBD) dataset 2019 estimated that nearly 800 million children worldwide have unsafe levels of lead in their bodies, with more than 50% living in Southeast Asia.2 The Institute for Health Metrics and Evaluation (IHME) estimated that in 2019, lead exposure accounted for 900,000 deaths, and 21.7 million years of healthy life lost (disability-adjusted life years) worldwide, due to long-term effects on health, with the highest burden in low- and middle-income countries.3 Young children, particularly toddlers, are vulnerable to lead exposure and toxicity due to their hand-to-mouth behaviors, and increased absorption rates. This case report aims to illustrate how lead encephalopathy can clinically resemble other encephalopathic conditions, such as acute disseminated encephalomyelitis (ADEM), potentially leading to oversight and delayed diagnosis.
CLINICAL DESCRIPTION
A 14-month-old boy with normal birth and development, relatively asymptomatic, had one episode of afebrile generalized tonic-clonic seizure as an 11-month-old, when awake. The seizure lasted for a duration of 5–10 minutes, and subsided without active intervention, followed by postictal drowsiness. The child was stabilized at a local hospital and was administered anti-epileptics intravenously. Brain MRI revealed no pathology. One week later, the child had another episode of afebrile seizure during feeding. He was admitted to hospital, where he was started on intravenous (IV) anti-epileptics and antibiotics. A repeat brain MRI suggested ADEM. Electroencephalography (EEG) was reported to be normal. Lumbar puncture report showed a cell count of 4 /µl (100% lymphocytes), 24 mg/dL of protein, 90 mg/dL of glucose, herpes simplex virus PCR negative, culture and sensitivity sterile. The child was discharged after 10 days on oral anti-epileptics. The child remained well for subsequent days but, 1 month later, developed vomiting (refractory to oral antiemetics) and intermittent irritability. He was admitted again, and a repeat brain MRI was done; the results were consistent with previous MRI findings. The child was referred to the authors’ centre due to persistent vomiting and irritability.
The child on examination was found to be mildly dehydrated, underweight, irritable, afebrile, had tachycardia (heart rate: 128 /min), pallor, and decreased muscle tone in both lower and upper limbs, plantar reflexes going down, and showed no signs of meningeal irritation.
On Day 1 of admission, the child was started on parenteral drug therapy, which included IV fluids (to maintain a urine output of 1 mL/kg/hour), ceftriaxone, and levetiracetam.
As vomiting was persistent and refractory to IV antiemetics, the child was started on tube feedings, and the pediatric gastrology team were involved. Gastrografin swallow was done to rule out gastroesophageal reflux disease, which revealed no gross abnormality.
On Day 2, an opinion of pediatric neurology was taken, given the child’s persistent irritability and history of seizures. The neurologist modified the child’s anti-epileptics, and suggested a repeat brain MRI and EEG for the child. Initial investigations, which were sent on admission, showed hemoglobin and total leucocyte levels to be at 6 g/dL and 9,870 /µl, respectively. A normal level of C-reactive protein, iron, and ferritin (iron: 119 mcg/dL, ferritin: 75.30 ng/dL) were noted. Peripheral smear showed anisopoikilocytosis with severe microcytic hypochromic anemia with basophilic stippling. Based on the peripheral smear report, the child was suspected to have lead poisoning. This led to the review of the child’s environmental history.
The father of the child works at a local paint factory in New Delhi, that uses lead in its manufacturing process. He often brought home painted toys from the factory for the child to play with, and observed the child chewed on the toys numerous times. Additionally, the child resided in an area with multiple small factories and ongoing construction works. The mother had observed the child ingest dust particles from these construction activities, which settled on the floors and windows of their home. Considering these potential lead exposure sources, a lead encephalopathy diagnosis was inferred. Diagnostic investigations included blood lead level (BLL) analysis, urine toxicology screening, urine coproporphyrin analysis, abdominal X-ray (to detect lead flakes), and X-rays of long bones (to identify lead lines).
MANAGEMENT AND OUTCOME
On Day 5 of admission, the urine toxicology screen was normal, but blood lead levels were grossly elevated (BLL: 71 mcg/dL). X-ray of the abdomen showed fecal loading; the X-ray of long bones showed dense metaphysis suggesting lead lines (Figure 1). Urine coproporphyrin was within the normal range.
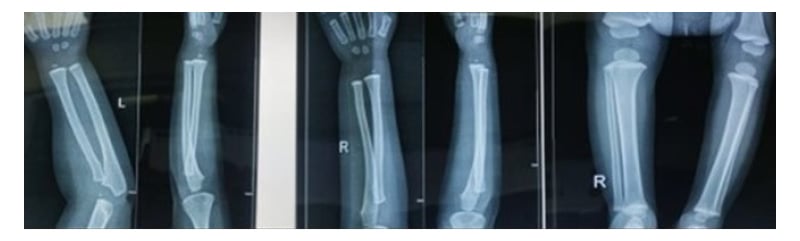
Figure 1: X-ray of long bones showing dense metaphysis suggestive of lead lines.
MRI of the brain with MR spectroscopy was suggestive of toxic encephalopathy, and EEG findings were consistent with cerebral dysfunction.
On Day 8 of admission, the repeat blood lead levels were 80 mcg/dL (Figure 2); hence, a plan was made to start IV chelation therapy. The child’s anemia was treated by transfusing packed red blood cells. The pre-chelation work-up included complete blood counts; serum electrolytes, including magnesium, urine routine and microscopy; G6PD levels; and liver enzymes.
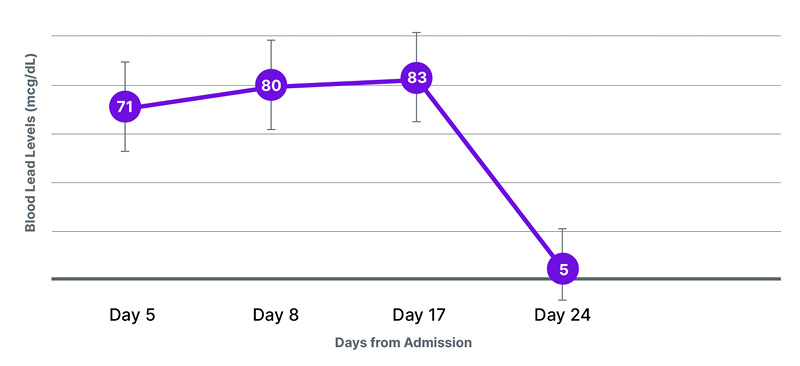
Figure 2: Trend of blood lead levels during hospital stay.
On Day 10, the pre-chelation work-up came back normal; thus, the child was started on dimercaprol intramuscular injection (5 mg/kg/dose), given after every 4 hours, for a total of 30 doses, and 50 mg D-penicillamine tablets, once a day, orally. Complete blood counts, liver enzymes, serum electrolytes, and urine routines were monitored every alternate day as per the standard treatment protocol.
On Day 3 of chelation therapy, the child became inconsolably irritable; the opinion of a pediatric neurologist and toxicologist was sought. Anti-epileptics were modified, and D-penicillamine was stopped. The blood lead level after the chelation therapy was still at 83 mcg/dL, so the child was started again on dimercaprol therapy for 5 days (30 doses).
Repeat blood lead levels after chelation therapy came down to 5 mcg/dL. The child was discharged on oral anti-epileptics and multivitamins, and was advised to attend regular follow-ups.
DISCUSSION
Lead encephalopathy is a severe but avoidable neurological disorder that is characterized by the toxic effects of lead on the central nervous system. It is a serious public health concern with global implications, particularly in low- and middle-income nations, where environmental lead poisoning is still a significant issue.
There are numerous sources of lead exposure attributable to its extensive utilization and environmental pollution, the most significant being the ingestion of lead-containing materials, which is especially prevalent among younger children. These sources encompass contaminated dust or soil, lead paint flakes, tainted food and spices, water passing through lead service lines, and lead-containing traditional medicines or foreign bodies.4 In India, the primary source of lead exposure often stems from contaminated lead dust and soil, and this is particularly prevalent in metro cities undergoing rapid construction. Additionally, numerous local paint manufacturing companies continue to incorporate high levels of lead in their manufacturing processes, resulting in the distribution of painted toys that easily find their way into homes with children.
While searching PubMed and MEDLINE, the authors found multiple cases of lead poisoning in children with a history of pica who ate paint peelings, herbal medicines, and lead-containing dust. In a case study from Africa, a 3-year-old girl diagnosed with lead encephalopathy had a history of pica, and consumption of herbal medications.5 In the authors’ case, the 14-month-old boy resides in New Delhi, where his father is employed at a local paint factory, utilizing lead in its paint manufacturing process. The father frequently brought home painted toys from the factory for the child to play with and observed on numerous occasions the child chewed on these toys. Another potential source of lead exposure for the child could have been household dust, which he might have ingested due to the habitual hand-to-mouth contact. As shown in previous studies, analyses of the dust samples obtained from residential areas in Delhi, and different parts of India, have revealed notably elevated lead levels.6,7.
Lead poisoning in children initially presents with vague symptoms like vomiting, and abdominal pain, mimicking viral illness. With continuous exposure, children can have systemic involvement, including cognitive delay, encephalopathy, and anemia. Children with lead encephalopathy exhibit symptoms that resemble other forms of encephalopathy, such as viral, autoimmune, or metabolic etiologies. As a result, diagnosis can be delayed or overlooked, leading to prolonged illness and increased health expenditure. The same was exemplified in the authors’ case, where the child initially received treatment for ADEM at multiple hospitals, before ultimately being diagnosed with lead encephalopathy at the authors’ hospital. After receiving the treatment, the child has been doing well.
Lead poisoning in an individual is diagnosed based on the presence of relevant clinical features and high blood lead levels. There are no known safe blood lead levels, and concentrations as low as 3.5 mcg/dL may cause decreased intelligence in children, behavioral difficulties, and learning problems.1 Other valuable investigations include a complete blood count and ferritin to identify iron deficiency anemia, a peripheral smear to look for basophilic stippling, raised urinary coproporphyrin, presence of lead flakes on abdominal X-ray, lead lines on X-ray of long bones, and neuroimaging to look for encephalopathic changes. Studies have shown that children with underlying iron deficiency anemia, and exhibiting pica, are more prone to lead toxicity.8
As per the World Health Organization (WHO), primary treatment measures for all children with suspected or confirmed lead exposure include source removal and correction of underlying anemia; while chelation therapy, either oral or parenteral, is recommended for children with BLL >45 mcg/dL.4 Primary chelators include injectable calcium disodium edetate (CaNa2EDTA) and oral succimer, along with injectable dimercaprol and oral D-penicillamine.9 During chelation therapy, it is essential to monitor complete blood counts, liver enzymes, serum electrolytes, and urine routine and microscopy. Children and adolescents with significant neurological features of lead toxicity or lead encephalopathy require urgent admission and parenteral chelation therapy.4
Prevention of lead exposure stands as the cornerstone in decreasing the incidence and mitigating the risks of lead poisoning among children. This necessitates a multidisciplinary approach, involving health workers, governmental bodies, and the general populace. Several countries have enacted legislation, limiting the quantity of lead in paints. Additional methods entail screening asymptomatic children for blood lead levels, conducting dust clearance tests to assess lead levels in household dust, regular maintenance of lead service lines transporting drinking water, and stringent quality regulations for traditional medicines in the market.10
In conclusion, lead poisoning continues to pose a significant threat in current times, and can prove fatal if not promptly identified; hence, it is imperative to conduct blood lead level screenings for children exhibiting nonspecific symptoms and a history of pica.
Furthermore, it is noteworthy that children displaying symptoms of lead encephalopathy may exhibit clinical similarities to conditions such as ADEM or viral encephalitis. Therefore, in cases where a child is suspected of having encephalopathy, and remains unresponsive to conventional therapies, lead poisoning should be considered as a potential differential diagnosis.







