Abstract
Galactose-α-1,3-galactose (α-Gal) is an oligosaccharide that was first described as a cause of immunoglobulin E-mediated anaphylaxis in cases of first-in-man reactions to the monoclonal antibody cetuximab. Soon thereafter, immunoglobulin E antibodies to this epitope were linked with anaphylactic episodes to mammalian meat, which had a characteristic delay of ~3-6 hours. The ‘α-Gal syndrome’ is now recognised globally as a significant form of food allergy, albeit with regional variation, which reflects that sensitisation relates to bites from certain species of hard tick. The α-Gal epitope is present in organs and muscles from most mammals (with the exception of humans, apes, and Old World monkeys) as a glycan conjugated to both proteins and lipids. There are a number of unusual features that distinguish α-Gal from other traditional food allergies, including the fact that the oligosaccharide can be causal in both immediate and delayed allergic responses, and that co-factors, such as alcohol or exercise, often relate to the instigation and/or severity of clinical reactions. In this narrative review, the authors focus on the novelty of α-Gal’s intrinsic lipid form; consider aspects of glycolipid digestion, absorption, and processing; and explain how this ‘glycolipid hypothesis’ may explain several of the clinical features of α-Gal syndrome. This review draws on pioneering studies of the biochemistry of α-Gal, contemporary understanding of lipid metabolism, and comparisons to other clinically important oligosaccharides.
INTRODUCTION
It has been 10 years since the blood group antigen galactose-α-1,3-galactose (α-Gal), an oligosaccharide produced by non-primate mammals, was identified as an important allergen epitope in immediate anaphylaxis to the monoclonal antibody cetuximab.1,2 While carbohydrates had been previously recognised as targets of immunoglobulin (Ig)E, this was the first example of a carbohydrate epitope that commonly contributed to severe clinical symptoms: i.e., anaphylaxis. In 2009, it was reported that α-Gal was an important allergen in episodes of delayed anaphylaxis to mammalian meat.3 As a result of reactions occurring to a variety of mammalian products, including meat, innards, and gelatin, this allergy is now commonly referred to as the ‘α-Gal syndrome’. To date, the authors are aware of published reports of α-Gal syndrome from North and Central America, Europe, Asia, Australia, Africa, and, anecdotally, from South America.4,5
When considering the immune response to α-Gal, it is important to realise that this is the same antigen that was initially described by Landsteiner and Miller6 as ‘B-like’. The relevance is that immunocompetent humans produce abundant natural antibodies (i.e., IgM, IgG2, and IgA, specific to α-Gal)7,8 presumably related to immune recognition of α-Gal-laden enteric Gram-negative bowel flora.9 An important feature of α-Gal is that it can be present as both glycoproteins and glycolipids,10,11 and that there can be significant diversity in the complexity of the oligosaccharide. For example, some glycoconjugates have a single terminal α-Gal epitope, but upwards of eight branches with terminal α-Gal have been reported.12,13 It is currently unclear whether this complexity affects epitope recognition in hypersensitivity reactions.
Remarkably, it has become clear that bites from certain hard ticks are important agents for the induction of IgE specific for α-Gal.14 In the USA, the lone star tick (Amblyomma americanum) is the major contributor, but, in other parts of the world where there are no lone star ticks, other hard ticks have been implicated. A likely scenario is that factors in tick saliva favour the class-switch of pre-existing natural antibody-producing α-Gal-specific B cells. Indeed, it is well established that saliva from hard ticks can favour the production of host T helper 2 cell (Th2)-related cytokines.15,16 Two groups have now identified the presence of a carbohydrate with a terminal α-Gal moiety in hard ticks. Hamsten et al.17 reported α-Gal in the gut of Ixodes ricinus and, more recently, α-Gal was described in the saliva of Amblyomma sculptum.18 The latter report by Araujo et al.18 went on to demonstrate that tick saliva was sufficient to induce specific IgE to α-Gal using a humanised mouse model (i.e., an α-Gal knock-out mouse), further bolstering the argument that ticks are an important cause of IgE sensitisation to α-Gal. While these experiments suggest that some hard ticks intrinsically produce α-Gal, an alternative possibility is that ticks could harbour bacterial symbionts that are the source of the α-Gal. The latter possibility is interesting in light of the fact that α-Gal syndrome appears to be uncommon in some areas of the south-eastern USA that are reported to have established lone star tick populations;19 however, an alternative explanation may be that tick populations have been dynamic, and existing tick maps rely on historical data.20 In any event, the mechanism whereby tick bites favour IgE induction remains an important but open question, as well as the possibility that there are other mechanisms of sensitisation. Taken together, the α-Gal syndrome has a number of features that are unusual for an IgE-mediated allergy (Box 1A). This article reviews the evidence that both immediate and delayed forms of anaphylaxis to mammalian products can be mediated by IgE specific to α-Gal and speculates on the mechanisms that explain this discrepancy.
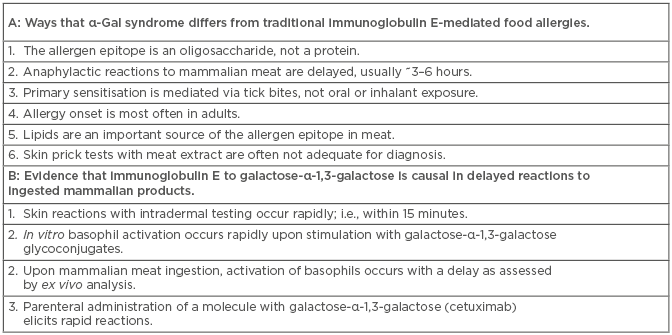
Box 1: A) Ways that α-Gal syndrome differs from traditional immunoglobulin E-mediated food allergies. B) Evidence that immunoglobulin E to galactose-α-1,3-galactose is causal in delayed reactions to ingested mammalian products.
EVIDENCE THAT IMMUNOGLOBULIN E IS CAUSAL IN DELAYED ANAPHYLAXIS TO RED MEAT
It is well established that the mammalian meat allergy related to α-Gal typically has a delayed response: i.e., the reactions occur >2 hours after exposure.21 This was apparent from the initial cases that were identified in south- eastern USA and was confirmed in prospective controlled food challenges. Ten of twelve adult subjects with detectable specific IgE to α-Gal and self-reported history of reactions to meat experienced a hypersensitivity reaction following a monitored challenge with 150 g of pork or beef sausage.22 None of the reactions were evident before 2.5 hours and the mean time to reaction was 4 hours and 12 minutes. Moreover, clinical reactions were largely mirrored by the time course of ex vivo basophil activation, which peaked at 4 or 5 hours post challenge.22 The time course of reactions to mammalian meat clearly differs from those that occur to cetuximab, which typically occur within the first 30 minutes of the first antibody infusion.1,23 Notably, very few patients who have had reactions to cetuximab attempted a second dose.
There are several lines of evidence that support specific IgE as being causal in the delayed reaction that occurs in α-Gal syndrome (Box 1B). Perhaps the most compelling is that in vitro stimulation of α-Gal sensitised basophils, with cetuximab or α-Gal-laden glycoproteins, elicits rapid stimulation as judged by CD63 expression (i.e., within 30 minutes).22,24 This is in contrast to the delayed kinetics of basophil activation examined ex vivo from peripheral blood mononuclear cells drawn following oral meat challenge. The working hypothesis to explain how IgE sensitisation to α-Gal could mediate delayed reactions to mammalian meat relates to the time required for digestion, absorption, processing, and presentation of glycoconjugates in a form that could be recognised by IgE bound to the surface of tissue mast cells or circulating basophils. An explanation that seems likely is that the delay specifically involves the glycolipid forms of α-Gal. The idea that the causal mechanism involves immune pathways other than IgE cannot be excluded, although to date this has not been supported by direct evidence. For example, activation of CD4+ T cells by an oligosaccharide would be unexpected via traditional major histocompatibility complex II, except in the case of zwitterions. Non-canonical presentation via CD1 molecules to T cells or natural killer T cells remains a possibility, but has been little explored.25,26 Another possibility is that specific IgG1, an immunoglobulin subtype that increases in parallel with α-Gal specific IgE, could play a role by activating FcγR-expressing haematopoietic cells.27,28 Taken together, existing data strongly supports a role for specific IgE to α-Gal as causal in delayed reactions to mammalian meat, but further research may uncover additional immune cells and molecular mediators that are also important.
THE GLYCOLIPID HYPOTHESIS
The fact that α-Gal is an oligosaccharide is often considered the most important feature of this allergen. However, it could be argued that an equally distinguishing feature of the α-Gal allergen is that it exists in the form of a glycolipid. Indeed, while there are many examples of carbohydrate allergens and of lipids modulating Th2 cell immune responses, the authors are unaware of any other common food allergen that is intrinsically part of a lipid.29,30 Thus, it is important to consider the glycolipid content of mammalian meat and organs, as well as the biochemical pathways involved in glycolipid digestion and metabolism, when considering the pathophysiology of α-Gal allergenicity.
The first report of lipids with the terminal α-Gal epitope date back to studies of rabbit red blood cells in 1968.31 Subsequent experiments established that glycosphingolipids from almost all non-primate mammals were a rich source of α-Gal antigen.32 Neutral glycosphingolipids are the major form of glycolipids that have been reported to have terminal α-Gal epitopes, although gangliosides (negatively charged glycosphingolipids) can also have the epitope (Figure 1).10,33 Collectively, glycosphingolipids constitute a diverse family of membrane-bound lipids with a number of biological functions. The authors are unaware of studies that have characterised the amount of α-Gal linked glycosphingolipids in red meat, but it is clear that erythrocytes, which are present in the highly vascularised muscle tissue, are an abundant source.10 Bovine and porcine kidneys have also been shown to have glycosphingolipids with α-Gal.33
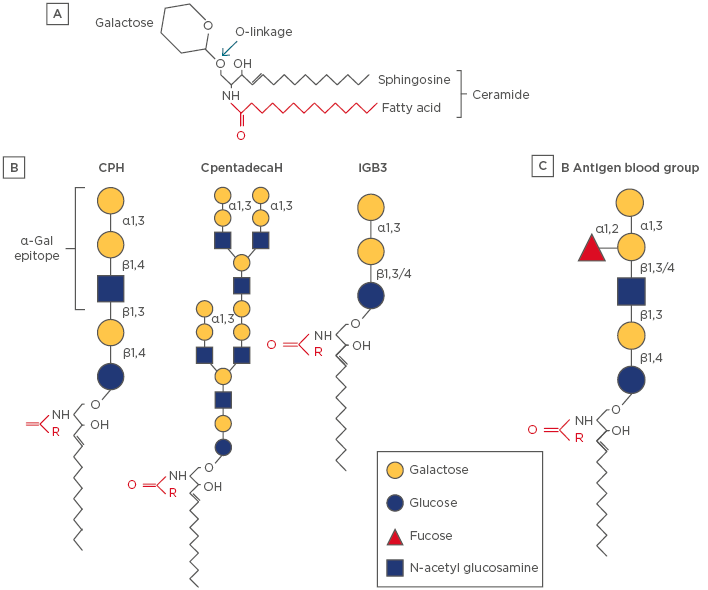
Figure 1: Schematic diagram of representative glycosphingolipids.
A) The backbone of the molecule is sphingosine (shown in black), which is amide-linked to a fatty acid (shown in red), which together forms a ceramide. The fatty acid tail can vary in length (14–36 carbon atoms) and degree of saturation. A glycosphingolipid consists of a ceramide coupled to a carbohydrate headgroup via an O-linked glycosidic bond. There are a large number of subspecies of sphingolipid and glycosphingolipids, which reflects a diversity of biologic functions.33 B) Examples of α-Gal-linked glycosphingolipids that have been characterised in mammalian (nonhuman) cells and tissues. Note that iGB3 has a terminal galactose-α-1,3-galactose motif but lacks N-acetylglucosamine. The full α-Gal epitope is often considered to be the trisaccharide form, including N-acetylglucosamine; however, many anti-α-Gal antibodies can recognise the disaccharide.26 C) The blood group B antigen is a glycosphingolipid that is structurally homologous to CPH.
α-Gal: galactose-α-1,3-galactose; CpentadecaH: ceramide pentadecahexoside; CPH: ceramide pentahexoside; iGB3: isogloboside 3.
Specific information about dietary glycosphingolipid digestion and systemic absorption is limited and is largely derived from studies using animal models. For major dietary lipids (i.e., triglycerides) hydrolysis by pancreatic enzymes in the intestinal lumen generates fatty acids and glyceride metabolites. Subsequently these enter intestinal epithelial cells, facilitated by bile salts, in the form of micelles. The lipid constituents are then metabolised and packaged into chylomicrons. These large lipoprotein particles are then released into the lacteals where they subsequently traffic to the systemic circulation via the thoracic duct.35 Once in the bloodstream, lipids pass to other lipoprotein particles, including very low-density lipoproteins, low-density lipoproteins (LDL), and high-density lipoproteins (HDL), and ultimately to end-organs, such as the liver, and to adipose or muscle tissue. The key point is that the time frame for lipids to pass from the gut to lipoprotein particles small enough to penetrate the microvasculature or tissue (i.e., LDL or HDL), and thus be recognised by specific-IgE bound to the surface of basophils or mast cells, is expected to occur on the order of hours (Figure 2). Indeed, Labbé et al.36 reported that ingestion of a radiolabelled lipid in human volunteers with positron emission tomography/computed tomography (PET/CT) imaging revealed that peak levels were achieved in the distal thoracic duct at 4 hours and in muscle at 5 hours. Thus, this provides a rational mechanism that explains why ingested forms of α-Gal-linked glycolipid could lead to an IgE-mediated reaction with delayed onset. While this remains a favoured explanation, there are additional aspects to this ‘glycolipid hypothesis’ that warrant consideration.
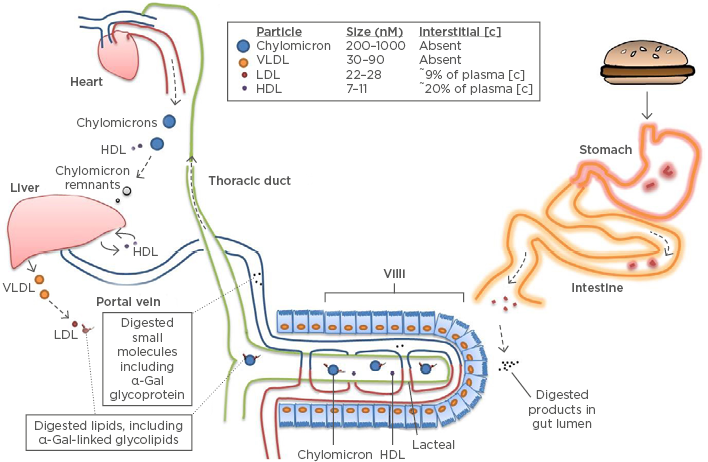
Figure 2: Model of delayed anaphylaxis to red meat.
The α-Gal syndrome and the ‘glycolipid hypothesis’. Epitopes containing α-Gal are consumed as glycoproteins and glycolipids in mammalian products. Neutral glycosphingolipids account for most of the α-Gal-bearing lipids.10 The mechanism and efficiency of transit through the epithelial barrier is unclear and may involve passive or active processes. Glycolipids are packaged into chylomicron lipoprotein particles, although incorporation into HDL within the intestine is also possible.34 These lipoprotein particles transit via the lacteals into the thoracic duct before entering the systemic circulation at the left subclavian vein. Lipids can only filter into interstitial tissue after passing to the relatively smaller LDL or HDL particles.34 Peak levels of lipids emerge from the thoracic duct ~4 hours post-prandial.35
α-Gal: galactose-α-1,3-galactose; HDL: high-density lipoprotein; LDL: low-density lipoprotein; VLDL: very low density lipoprotein.
A fundamental question remains: how do α-Gal linked glycosphingolipids access the systemic circulation? Current evidence suggests that dietary glycosphingolipids cannot pass directly from the lumen of the intestine to the systemic circulation with the carbohydrate linkage intact. In experiments published in 1969 that used radiolabelled cerebrosidase and a rat feeding model, Nilsson37 concluded that dietary glycosphingolipids are metabolised in the small intestine and are not transported intact into the thoracic duct lymph. However, the α-Gal syndrome offers clear evidence that, at least in some subjects, ingested α-Gal epitopes can pass through the intestinal epithelium. Moreover, the barrier problem is not unique to α-Gal. Most ingested protein or carbohydrate macromolecules require digestion before the metabolites can transit into and through the epithelial barrier, but in the case of all IgE-mediated food allergies, allergens with intact epitopes are clearly penetrating the epithelial barrier. Possible explanations include transcellular or paracellular transit, which could involve antibody-mediated processes. For example, there is evidence that the low affinity IgE receptor, FcεRII (also known as CD23), on apical enterocytes can facilitate the transit of specific allergens from the lumen to the lamina propria.39 The mechanism that explains how α-Gal glycolipids are packaged into lipoprotein particles within the intestine is unknown. While chylomicrons are the dominant particles that shuttle dietary lipid to the systemic circulation, direct incorporation into HDL in the intestine is also possible.35,40 Intersubject variability in epithelial transit could be a factor that impacts which of the sensitised individuals develop allergic symptoms (many subjects who are sensitised can tolerate red meat) and/or the severity of allergic symptoms, a possibility that fits with the premise that allergy is an epithelial barrier disease.41 Galili et al.13 suggested that a typical hamburger may contain up to or exceeding 100 billion α-Gal epitopes, even a small fraction of this total could yield a significant antigenic load.13 Intersubject differences in lipid metabolism could also impact the time taken for the allergen to access peripheral tissue and also the severity of reactions. For example, fatty acids have been shown to transit to the liver and muscle more rapidly following a meal in subjects with Type 2 diabetes mellitus than in healthy controls.42
LESSONS FROM OTHER OLIGOSACCHARIDES
Recent reports have highlighted other oligosaccharides as the target of IgE-mediated food allergy.43 Galacto-oligosaccharides (GOS) represent a mixture of unconjugated oligosaccharides (i.e., those with no protein or lipid backbone) with a series of terminal galactose residues that are commonly used as prebiotics. The mechanism of sensitisation is unclear, but several cases of anaphylaxis to GOS have been identified in Asian children.44 Importantly, the relevant epitopes do not appear to have the α-1,3 linkage and thus the antibodies to GOS, despite being specific to galactose residues, are distinct from those in α-Gal. A key difference in the clinical response to GOS and α-Gal is that the former has been reported to occur much more rapidly. In the initial 2012 GOS report, all the cases occurred within 30 minutes.44 Thus, this example provides further evidence that the backbone of the α-Gal glycoconjugate is the key to understanding the characteristic delay.
N-glycolylneuraminic acid (Neu5Gc) represents a glycan with xeno-antigen characteristics that are similar to α-Gal. Neu5Gc is a sialic acid that is widely expressed in mammals but is not endogenously produced in humans owing to a mutation in the gene that encodes the enzyme cytidine monophospho-N-acetylneuraminic acid hydroxylase. This mutation, estimated to have occurred 2–3 million years ago in ancestral humans, prevents Neu5Gc generation from the precursor sialic acid N-acetylneuraminic acid.45 Similar to α-Gal, many humans develop IgG antibodies specific for Neu5Gc, and, interestingly, a role for the immune response to Neu5Gc in the pathophysiology of carcinogenesis and inflammatory diseases has been suggested.46 However, a key difference between the two xeno-antigens is that Neu5Gc has been shown to be metabolically incorporated into human cells and tissues following dietary intake.47 There is no simple explanation that would allow for a similar process in relation to α-Gal. The difference relates to the fact that in the case of Neu5Gc, the enzyme defect is at the level of sialic production, whereas with α-Gal it is with the carbohydrate linkage. Additionally, IgE specific for Neu5Gc has not been reported.48
GALACTOSE-α-1,3-GALACTOSE–BEARING GLYCOPROTEINS
Glycolipids have been little studied in relation to the α-Gal syndrome, though multiple investigators have reported on glycoprotein sources of α-Gal in mammalian meat or organs. Takahashi et al.49 studied Japanese subjects who reported anaphylaxis to mammalian meat and identified laminin-γ1 and collagen α1 as α-Gal-containing glycoproteins. Apostolovic et al.38 investigated a Swedish cohort and found seven novel α-Gal-containing beef allergens. Of these glycoproteins, creatine kinase M-type, aspartate aminotransferase, β-enolase, and α-enolase were heat-stable. A more recent report identified α-Gal on angiotensin-I-converting enzyme and aminopeptidase N in porcine kidneys, and additionally showed these glycoproteins could trigger rapid in vitro basophil activation on cells collected from subjects with α-Gal syndrome.24
ADDITIONAL FACTORS THAT MAY MODULATE ALLERGIC REACTIONS TO GALACTOSE-α-1,3-GALACTOSE
Several groups have described co-factors that are associated with clinical reactions to α-Gal. Two of the studies that focussed on subjects with reactions to porcine kidney identified co-factors in >70% of the cases, with alcohol consumption being the most common, followed by exercise.50,51 These data suggest that co-factors, especially alcohol, may be more important in α-Gal syndrome than in other forms of food allergy.52 The mechanisms whereby alcohol or exercise contribute to a lower threshold of allergic reactions are not entirely clear, but could relate to decreased epithelial barrier function or to sensitisation of the calcium ion channel that facilitates histamine-mediated reactions (i.e., transient receptor potential vanilloid 1 receptor).53 A particularly interesting possibility is that alcohol is important in α-Gal syndrome because of a relationship between alcohol and lipid metabolism. Indeed, a century ago, the effects of acute alcohol ingestion were described by Feigl54 as lipaemia of intoxication and more recent studies have demonstrated that alcohol raises post-prandial levels of plasma triglyceride and HDL.55,56
The form of mammalian product ingested may also affect the likelihood and/or severity of allergic reactions related to α-Gal. Porcine kidney seems to elicit reactions with more rapid kinetics than mammalian meat. For example, Morisset et al.50 described 14 subjects with IgE to α-Gal who had a history of anaphylaxis or urticaria to porcine kidney, and 64% of the reactions were reported within 2 hours. A similar result was reported by Fischer et al.,51 where 13 of 21 subjects with a history of reactions to porcine kidney relating to α-Gal occurred within 2 hours. There are multiple possible explanations to consider, one of which relates to the relative content of glycoprotein versus glycolipid containing α-Gal epitopes. However, it is clear that α-Gal containing glycolipids are present in porcine kidney,33 and the favoured explanation is that the content of α-Gal glycoconjugates is higher in kidneys than meat (i.e., muscle tissue). Using an inhibition enzyme-linked immunosorbent assay (ELISA), Morisset et al.50 showed that porcine kidney was a much stronger inhibitor of monoclonal antibody binding (specific to α-Gal) than either pork or beef. Two other reports showed a similar result using prick-to-prick testing, where porcine kidney led to more frequent positive results and/or larger wheals than beef or pork.24,51
BEYOND ALLERGY
Because α-Gal represents an epitope that is regularly consumed in the form of mammalian glycolipids and a subset of the population has a strikingly different type of immune response to the antigen, it is possible that IgE to α-Gal, or concomitant specific IgG1 or T cell production, has implications beyond traditional allergic disease.27,28,57,58 It is intriguing that red meat and high fat dairy are also risk factors for another inflammatory disease with geographic variability, and that has been associated with elevated total IgE levels and mast cells: i.e., in atherosclerosis.59-62 Consistent with this possibility, the authors have recently reported that IgE to α-Gal was associated with the severity of coronary artery disease in a cohort of ‘at-risk’ adults from Virginia, USA, whose recruitment was unrelated to allergic disease.63 Prior associations of IgG antibody response to α-Gal have been described for thyroid disease and inflammatory bowel disease.13
CONCLUSION
One of the striking aspects of α-Gal, an oligosaccharide that can be present on both mammalian glycoproteins and glycolipids, is that it contributes to two forms of anaphylaxis with markedly different kinetics. Multiple lines of evidence point to a role for IgE in mediating these reactions. It is possible that the glycolipid form of α-Gal, which is unique for an allergen, is critical to understanding the characteristic delay. Investigation into α-Gal-bearing glycolipids may shed further insight into aspects of this atypical food allergy but may additionally illuminate connections with inflammatory diseases not traditionally associated with allergy.






