Speakers: Adelaide Ann Hebert,1 Giovanni Pellacani,2 Giuseppe Micali3
1. Chief of Pediatric Dermatology, UTHealth McGovern Medical School in Houston, Texas, USA
2. Chairman of Dermatology and Dean of the Faculty of Medicine, University of Modena and Reggio Emilia, Modena, Italy
3. Head of Dermatology and Venereology, University of Catania, Catania, Italy
Disclosure: The speakers received speaker fees from RELIFE S.R.L. (Menarini Group) and have declared no conflicts of interest outside the submitted work.
Acknowledgements: Writing assistance was provided by Dr Eleanor Roberts, Beeline Science Communications, Ltd, London, UK.
Disclaimer: The opinions expressed in this article belong solely to the named speakers.
Support: The writing and editorial support was funded by RELIFE S.R.L. (Menarini Group).
Citation: EMJ Dermatol. 2021;9[Suppl 4]:2-9.
Meeting Summary
Dermatitis, including atopic dermatitis (AD), contact dermatitis (CD), and diaper dermatitis (DD), manifests as a red, itchy, flaky, inflammatory skin reaction. External factors, including antigens, allergens, and irritants, can penetrate the skin barrier, especially if it is already disrupted and broken. Combined with internal factors, such as a genetic predisposition to dermatitis, external factors can set up an inflammatory cascade and result in acute, subacute, and chronic skin conditions. Recent findings point to a role for oxidative stress in dermatitis as the generation of oxidants can both cause and exacerbate skin barrier disruption and inflammation. Therapies targeting various forms of dermatitis can range from basic lifestyle advice, such as limiting contact with known exacerbating factors and avoiding long, hot baths or exposure to cold temperatures, to the use of emollients. Such emollients are generally formulated with an oily phase to restore lipids in epidermal layers and improve skin barrier function. Basic formulations may also contain urea or other substances able to hold more water within the skin. In recent decades, a new generation of emollients have been proposed (emollients ‘plus’) with the addition of active substances able to act against pathogenetic mechanisms of dermatitis or improve cellular restoration. For example, the recently developed, oxidant-targeting ingredient furfuryl palmitate represents a new option in the treatment of eczematous dermatosis. These emollients plus can be used alone or in combination with therapies such as corticosteroids.
In this series of three talks, Prof Pellacani discussed pathogenesis and management of AD and CD, Prof Hebert talked about the role of oxidative stress in AD and CD and their treatments, and Prof Micali shed some light on the continued use of urea as an ingredient in dermatitis-targeting therapies.
Introduction
Eczematous dermatitis (or eczema) includes AD, CD, and DD. This inflammatory skin reaction presents as erythema, vesicles, desquamation, and itch.1 Dermatitis usually first occurs in childhood with approximately 10% of children affected.2,3 Occurrence can have a significant burden on a person’s quality of life, restricting social, educational, and work-related activities, especially in more severe cases, and interfering with sleep. There can also be emotional and psychological burdens associated with pain and irritation and the social stigma of the skin’s appearance.3
The basis of treatment for most types of dermatitis is the use of emollients.4 These may include additional active factors such as furfuryl palmitate, which targets oxidative stress-related pathways in the skin,5 and urea, which is utilised for both its humectant and keratolytic properties, dependent on concentration, for a variety of skin and nail disorders.6
In this series of three talks which outlined the manifestation and treatment of a variety of forms of dermatitis, Prof Pellacani talked about ‘Evidence-based management of atopic dermatitis and contact dermatitis’, Prof Hebert discussed ‘New horizons in paediatric dermatology disease’, and Prof Micali shed some light on ‘Urea: a century of proven clinical efficacy in dermatology’.
Pathogenesis of Atopic Dermatitis and Contact Dermatitis
AD and CD present two main phases, explained Prof Pellacani: “an acute phase, which is characterised by spongiosis and exocytosis and gives rise to intense erythema, papule/vesicles, crusts, itch, and burning sensations, and a chronic phase where inflammation decreases and there is thickening of the epidermis with acanthosis/parakeratosis, desquamation, and lichenification.”
The pathophysiology of extrinsic AD, encompassing both acute and chronic phases, is influenced by skin barrier defect, genetic predisposition, and the environment.7 The skin barrier is formed of stratum corneum (the outer layer of the epidermis comprising differentiated keratinocytes) and epidermal layers, with keratinocytes interconnected with tight junctions between epidermal cells.6,8 Defects in the skin barrier, according to Prof Hebert, are “the cornerstone and an initiator of the pathogenesis of AD.”
Skin barrier dysfunction can arise from both environmental factors, such as exposure to chemicals, and internal influencers. For example, one of the players in skin barrier dysfunction is mutations in the gene coding for the protein filaggrin, which is abundant in the stratum corneum. Loss of filaggrin can lead to structural defects at this layer of the epidermis due to its role in forming the protein–lipid matrix that helps to maintain skin pH and hydration.8
In AD and CD, as a consequence of the barrier defect, skin permeability is increased in both directions. There is loss of water from inside to outside (as measured by transdermal water loss), responsible for dry skin and increased permeation of substances from outside to inside, including irritants and antigens, resulting in the inflammatory immune system reaction.8,9 Such reactions lead to the experience of pruritus, which can exacerbate both skin barrier dysfunction and entrance of external substances, and promote cytokine release and epidermal thickening, leading to chronic disease status.8
The interplay between the trinity of barrier defects, allergy/immunology, and pruritus results in upregulation of a number of immune system cells and inflammatory cascades. Prof Pellacani reported that while it is known that “CD and AD present different pathways, different signals, and different actors,” it has to be kept in mind that, for both conditions, “we have an altered barrier, with lipid unbalance, that generates increased permeability. An inflammatory response is then generated as a consequence of substance penetration and immune system activation. The oxidative stress caused by inflammation is only partially counteracted by the skin’s antioxidant capacity and this release of inflammatory mediators is responsible for the oxidative damage of the tissue.”
In AD, penetration of antigens activates Th2 cells, via Langerhans and dendritic cells, to produce inflammatory cytokines, including IL-4 and IL-13. Inflammatory signals can then recruit mast cells, T cells, and leukocytes from the blood. These subsequently induce release of other cytokines, such as IL-17, IL-22, TNF-α, and interferon-γ, along with histamine. A similar pathway is shown in allergic CD, though there can be wider cytokine involvement and more direct recruitment of Th1, Th2, and T-regulatory cells from the blood. In irritant CD, innate immune system cells play more of a role via direct recruitment of neutrophils, lymphocytes, macrophages, and mast cells, which contribute to the inflammatory cascade.10
Oxidative Stress in Atopic Dermatitis
“We look at the role of oxidative stress as a new construct with regard to various disease states in the realm of medicine,” reported Prof Hebert. Oxidative stress is defined as ‘the formation of oxidants in the cells of the human body that acutely or chronically exceeds the antioxidant defence capacity’.11 These oxidants include free radicals such as superoxide, nitric oxide, and hydrogen peroxide.11,12 Oxidative stress is implicated in myriad medical conditions including cardiovascular disease, diabetes, neurodegenerative disorders, inflammatory disease, and cancer11 and, according to Prof Hebert, has now been recognised as playing a part in AD.5
While the concept of oxidative stress in AD has been around for a few decades, Prof Hebert highlighted how “it has come to the forefront more recently as we better understand the pathogenesis of this disease state overall.” Oxidative stress can play a role in, and can be generated by, the previously discussed trinity of skin barrier abnormalities, allergies/immunology, and pruritus (Figure 1).5,11
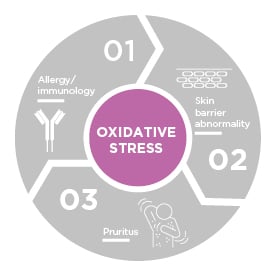
Figure 1: Interplay among oxidative stress, skin barrier defect, inflammation, and pruritis in atopic dermatitis.
Adapted from Hebert et al.5
In the skin, free radicals are mainly produced by leukocytes, macrophages, and mast cells when recruited and activated in the tissue by mediators (e.g., cytokines or growth factors). Environmental factors, such as air pollutants, ultraviolet radiation, volatile organic compounds, formaldehyde, and cosmetics can also be a source of oxidative stress.11-13 Oxidative stress promotes tissue inflammation as a result of its role in upregulation of proinflammatory cytokines and pathways involving the DNA transcription protein complex NFκB.12 Free radicals and other oxidative stress components can damage epidermal keratinocytes at the level of DNA and cellular enzymes, as well as through damage to the cell membrane through lipid oxidation. This can result in both cell damage and cell death, as well as in further upregulation of inflammatory cells and subsequent free radical release (Figure 2).11,13,14
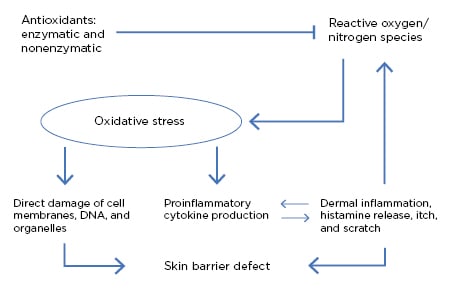
Figure 2: Oxidative stress and its role in skin barrier defects and inflammation.11
Oxidative stress “plays a key role in worsening the barrier defect in AD,” explained Prof Hebert, who stressed that recognition of the role of oxidative stress in AD pathogenesis is important to understand that “there is damage to cellular structures, resultant inflammation, and defects in the skin barrier that won’t repair.”
Diagnosis of Dermatitis
To be able to treat a patient with AD, Prof Pellacani highlighted that “we need to know the specific factors that cause the disease.” He explained: “intrinsic factors, absolutely required in AD development, should also be taken into account in allergic CD since subjective characteristics can influence disease insurgence and manifestation.”
As such, diagnosis should include the study of possible allergens and environmental conditions directly involved in the pathogenesis of the disease. A detailed personal and family history needs to be taken, including ascertaining exposure to environmental irritants (e.g., at a person’s workplace), alongside execution of serologic and contact tests (IgE).4,15
Treatment of Atopic Dermatitis and Contact Dermatitis
Treatment of AD and CD may vary according to disease phase and severity. A baseline approach for tackling these conditions is targeting skin barrier dysfunction.4 To tackle both acute and chronic AD and CD, there should also be an avoidance strategy. This includes recognising and steering clear of any substance that can favour disease insurgence, such as physical factors (hot water, a cold/dry environment), mechanical factors (wool), chemicals (acids, bleaches, solvents), biological factors (allergens, microbes), and volatile factors (tobacco smoke, traffic exhaust, pollution). Dietary factors to avoid are only those that are specific to the patient. These steps may help reduce the impact of substances on the skin reaction.4,15,16
There are some basic skin care practices Prof Pellacani suggested to help keep the skin barrier intact and prevent dehydration. These include using a delicate skin cleanser with nonirritant and hypoallergenic formulations, using any detergents for only a short period of time (2 minutes), and having short, lukewarm showers or baths (27−30oC).
Regular application of emollients or moisturisers on the skin is, according to Prof Pellacani, “the mainstay of management” for AD and CD, with strong recommendations in guidelines for all levels of dermatitis severity.4,15-18 Emollients can help restore skin barrier function, promote epidermal hydration (when combined with an humectant), and reduce evaporation (when combined with an occludent).4,17 Basic emollients have been shown to improve mild-to-moderate dermatitis,4,15-18 with the advantage of a steroid-sparing effect when applied regularly and in adequate amounts.4 However, the use of emollients on eroded and inflamed skin may be poorly tolerated in the acute phase of AD or CD.4,16,17
Today, there are topical formulas containing proactive, nonmedicated substances that can help repair the skin barrier and reduce skin inflammation. Such additions include vitamins, flavonoids, saponins, bacterial lysate, and antioxidants.4,16
The next treatment step would be to consider topical anti-inflammatory treatments such as Class II or Class III corticosteroids, for which efficacy has been shown.4,16,17 Prof Pellacani divulged how the selection of a suitable corticosteroid should be made on the basis of the localisation of skin lesions as well as dermatitis severity and acuteness, while bearing the therapeutic index in mind. Following topical corticosteroids, topical calcineurin inhibitors can be used. For those with moderate-to-severe AD or CD, other therapies of consideration include phototherapies and systemic therapies.4,16,17
Emollients That Target Oxidative Stress
“We know that oxidative stress plays a key role in the aetiology of AD,” said Prof Hebert. There is, therefore, “an important scientific rationale to using an emollient device enriched with antioxidants.” Prof Hebert reported how addressing and treating oxidative stress effectively has become “paramount as part of our therapeutic strategy.”5 One such ingredient is furfuryl palmitate, an ester known to limit production of singlet oxygen, such as is found in reactive oxygen species.21 Furfuryl palmitate has been shown to provide therapeutic benefits in enhancing the skin’s barrier to permeability in people with mild-to-moderate AD.5,18-21
“These are innovative processes in the overall strategy in managing AD,” said Prof Hebert. Such emollient devices “really do have an impact in reducing inflammation and effectively restoring the skin barrier.”5 The use of furfuryl palmitate or its derivatives as emollient-based targeted agents can also reduce the need for steroids, particularly topical formulations, when managing AD.5,21
Prof Pellacani also has considerable experience with using emollient devices enriched with antioxidants, including furfuryl palmitate and vitamin E. For example, in an open-label, uncontrolled trial carried out at the Dermatology Department of the University Hospital of Modena, Modena, Italy, he showed clinical results following use of Relizema™ cream (RELIFE S.R.L. Menarini Group, Firenze, Italy; marketed formulation) by a group of 40 adult patients with mild-to-moderate AD or CD (data on file).
In this study, following 28 days’ twice-daily administration, statistically significant reductions were found on the primary endpoint, Investigator Global Assessment (IGA) scores (rated on a 0−4 scale, where 4 is ‘severe’), from the baseline mean score reflecting mild-to-moderate disease, to a mean score of ‘almost-clear-to-mild’ at Day 14, and ‘almost clear’ at Day 28. Similar improvements were found at Days 14 and 28 on the secondary endpoints, including eczema severity (as calculated with the Eczema Area and Severity Index [EASI]) and pruritus intensity (as evaluated with a visual analogical score). Patients also reported a statistically significant improvement in quality of life at the end of the trial (as evaluated with the Dermatology Life Quality Index [DLQI]).
Overall, it was found that skin condition improved in over 90% of patients on both investigator-rated and patient-rated assessments. Additionally, no safety issues were identified and most patients reported satisfaction with the product characteristics and ease of use.
Treatment of Diaper Dermatitis/Nappy Rash
DD (also termed diaper rash, napkin dermatitis, or nappy rash) is the most common skin eruption in infants and toddlers.22 DD is most often a form of irritant CD with four main pathological components in its development: natural skin barrier defect because of the diaper, irritation, inflammation, and microbial contamination.23,24
While there are, discussed Prof Hebert, “several technological advances in diaper design and other components to lessen the severity of DD symptoms,” the unique environment for the skin in the perineal and perianal areas, being frequently both covered and exposed to pathogens/irritants from urine and faeces, can lead to symptoms recurring.23,24
To alleviate and protect from DD there are a number of barrier creams, including creams with zinc oxide, or repair creams, such as those containing dexpanthenol.22 Prof Hebert also suggested the use of creams with anti-inflammatory ingredients, such as taurine, or antimicrobial properties, such as zinc gluconate.
Prof Hebert presented the results of a clinical study, conducted by an independent dermatologic laboratory in Europe (Eurofins Scientific, Luxembourg City, Luxembourg). The study evaluated a range of skincare products from RELIFE S.R.L. including Relizema™ baby care cream and Relizema™ spray and go. Both are marketed formulas composed of zinc gluconate, taurine, dexpanthenol, and zinc oxide, with additional almond oil in the baby care cream. In clinical studies involving 20 infants and toddlers aged 3−36 months, in separate trials, with Fitzpatrick skin Types I−IV and slight irritation of the diaper area, both products were found to effectively reduce percentage of skin with erythema or irritation after 2 weeks of application, with added efficacy when used over 4 weeks (data on file).
The Use of Urea in Skin Diseases
Urea is a polar, hygroscopic molecule which arises in the skin due to the breakdown of the above-mentioned protein filaggrin.6 In the stratum corneum, urea makes up 7% of the components of the ‘natural moisturising factor’, which also includes free amino acids, pyrrolidone carboxylic acid, lactate, and sugars. As such, urea plays a key role in the maintenance of skin hydration.6,25
Urea has been used for more than a century in dermatology for wound healing and conditions associated with dry and scaly skin.6,26,27 Different concentrations of urea exert different actions. At low concentrations (≤10%), urea acts as a humectant that can attract and retain water, from both the dermis and external environment, in the stratum corneum. At higher concentrations (>10%), urea exerts a keratolytic action that can, Prof Micali reported, “induce protein conformational changes by breaking hydrogen bonds, [thereby] reducing cohesion of keratinocytes.”6,25
Urea is available in different formulations, such as creams, gels, foams, ointments, and nail lacquers, and different concentrations, from 2% to 50%, which together provide several alternatives to improve the management of skin conditions. While there is minimal chance of irritation at concentrations of 2−10%, the use of 40−50% formulations may lead to temporary redness, stinging, and burning.28
The use of urea has been investigated for myriad skin conditions, including AD and CD, and urea-based formulations are recommended in guidelines discussing emollient therapy for such.4,17 Prof Micali reported that a meta-analysis he led of 92 randomised controlled trials involving people with AD showed that “among different types of moisturisers, clinical effectiveness appears most well-documented for urea-based preparations.”29
Formulations containing urea have also been found to be useful for other skin conditions, including xerosis (dry skin), ichthyosis (dry skin displaying ‘fish scale’ properties), psoriasis, diabetic foot, onychomycosis, keratosis, seborrheic dermatitis, radiation-induced dermatitis, dystrophic nails, tinea pedis, and pruritus.30 For example, at low concentrations of 2−12%, one of the main uses of urea-based formulations is for ‘simple’ xerosis and xerosis-related disorders, characterised, according to Prof Micali, “by the inability of the stratum corneum to maintain hydration.” In a clinical study of foot xerosis, shown at a high incidence in people with diabetes, a 10% urea formulation used over 1 month brought lasting relief, even after discontinuation of use.31 In a study of ichthyosis, a 10% urea emulsion administered twice daily for 30 days led to significant clinical improvements in xerosis and itch, disappearance of the ‘scales’ on participant’s skin, and normalisation of furrow size and morphology.32
At medium concentrations of 15−30%, Prof Micali discussed how urea has proven useful “in cases of low-to-moderate hyperkeratosis involving large areas.” One study utilising a 30% urea foam showed significant improvements after 2 weeks’ use, as well as significant improvement of patient-reported outcomes such as quality of life.33 However, Prof Micali warned, at this concentration “we have to always balance the keratolytic action with skin irritation.”
High-concentration formulations can include 40−50% urea. Here, the keratolytic properties of urea can be utilised to treat smaller areas of hyperkeratosis. One study involving people with plaque psoriasis with an excessive hyperkeratotic component found that twice daily treatment over 3 weeks with a 50% urea paste led to clearance of hyperkeratosis in 92% of cases with no side effects; in this study, ultrasound was used to show an approximate 50% reduction in epidermal thickness.34
Conditions such as psoriasis, onychogryphosis, and pachyonychia, as well as the fungal infection onychomycosis, can lead to severe nail thickening requiring nail plate removal (avulsion). This can be carried out chemically using, for example, twice-daily application of a 40% urea paste over 3 weeks, wherein the nail is gently scaped with a spatula daily. Results show almost total avulsion can be achieved using this regimen.35
Urea can also be used as a penetration enhancer and Prof Micali highlighted that, by softening the nail bed, urea allows a greater penetration of topical antifungals, corticosteroids, and other drugs, improving treatment outcomes. Such combination regimens in onychomycosis may be superior to monotherapy.36
Conclusion
AD, CD, DD, and many other skin disorders can interfere greatly with a person’s quality of life. Lifestyle changes are often needed to help limit and control such problems with topical emollients recommended for all severities of these skin conditions as baseline therapy. The newer generation topical treatment may include active ingredients, such as the oxidative stress-targeting furfuryl palmitate or the historically used urea in an innovative formulation. Such treatments can help limit the need for corticosteroids and work in harmony with them.

![EMJ Dermatology 9 [Supplement 4] 2021 feature image](https://www.emjreviews.com/wp-content/uploads/2021/03/EMJ-Dermatology-9-Supplement-4-2021-feature-image-940x563.jpg)





