Interviewees: Philip Conaghan,1 Lee S. Simon2
1. Leeds Institute of Rheumatic and Musculoskeletal Medicine, UK, Leeds Institute of Rheumatic and
2. Musculoskeletal Medicine, University of Leeds, UK, and NIHR Leeds Biomedical Research Centre, UK SDG LLC, Cambridge, Massachusetts, USA
Disclosure: Conaghan reports consultancies or speaker fees from AbbVie, BMS, Eli Lilly, Galapagos, GSK, Merck, Novartis, Stryker, and UCB. Simon is a consultant for Eupraxia and Xalud including injectable therapies; has consulting fees from Affinergy, Astrazeneca, Abraxxis, Alpha Rx, NuvoResearch, Roche, Pfizer, Novartis, PLx Pharma, Hisamatsu, Dr Reddys, Avanir, Cerimon, Leerink Swann, Alimera, Nomura, Luxor, Paraexel, Bayer, Rigel, JP Morgan, Regeneron, XTL, Inmedix, Eupraxia, Fidelity, Extera, Wyeth, Asahi, Samumed, Metabolex, Shire, Anthera, Antares, Vical, Daiichi Sankyo, Flexion, AcelRx, Inotek, Gilead, Sandoz, Knopp, Abbott, Omeros, Jazz, Takeda, Teva, Zydus, Proprius, Alder, Cephalon, Purdue, EMDSerono, Altea, Talagen, Tigenix, Agenus, Forest, Genzyme, CaloSyn, pSivida, Horizon, Pozen, Eicos Sciences, Analgesic Solutions, Kowa, Array, JRX Biopharm, Imprimis, Dara, Genco, Neos, Durect, Sanofi, Lilly, Idera, Medac, Remedy, and Kiniksa.
Acknowledgements: Medical writing assistance was provided by Amanda Barrell, Brighton, UK.
Support: The publication of this article was supported by Eupraxia.
Disclaimer: The opinions expressed in this article belong solely to the named interviewees.
Citation: EMJ. 2022;7[3]:45-51. DOI/10.33590/emj/10023791. https://doi.org/10.33590/emj/10023791.
Interview Summary
Osteoarthritis (OA) is the fastest growing cause of disability worldwide, but, with few proven therapeutic options, it is an underserved condition. With increasingly ageing populations contributing to a rising global prevalence, this unmet need only threatens to worsen in the coming years. To date, researchers have tried and failed in their bids to develop new ways to treat the pain and loss of function that significantly impacts health-related quality of life (HrQoL) and leaves people vulnerable to accumulating disability and at risk of cardiovascular disease (CVD), comorbidities, and mortality. Now, a novel way to deliver one of the only proven interventions for pain and inflammation, corticosteroid injections, is on the horizon for knee OA. Slow-release formulations could possibly prolong the clinical benefit of a single injection from 6 weeks to 6 months, providing a new option to improve HrQoL for people with OA, and maybe even breaking the cycle of inflammation that likely contributes to progression.
In this key opinion leader article, Philip Conaghan and Lee Simon discuss OA’s significant Quality of life (QoL) and long-term health impact. They also outline the current, inadequate treatment landscape, and explain how slow-release corticosteroids could potentially help tackle a huge unmet medical need.
THE LONG SHADOW OF OSTEOARTHRITIS
OA is a chronic disease of the joints that causes pain, inflammation, and loss of function. The progressive condition, characterised by multiple tissue pathologies involving cartilage, bone, and synovium, can lead to disability that may necessitate joint replacement. Affected joints vary, but the most common site is the knee, followed by the hand and the hip.1 While younger people may experience trauma-initiated OA, it is primarily an age-related condition attributable to slow accumulation of structural damage over time.1 In line with ageing populations worldwide, its prevalence among older people is on the increase. Data from the 2019 Global Burden of Disease (GBD) study show a 113.25% increase in cases, from 247.51 million to 527.81 million, between 1990 and 2019. The same period also saw a 114.5% increase in years lived with disability caused by OA.1
OA is the fastest growing cause of disability worldwide,2 and the impact can be significant, said Conaghan. “Not everyone has severe symptoms, but roughly one-third will have severe symptoms with impact on QoL,” Conaghan told EMJ. “People have pain and stiffness in their joints, and sometimes this pain and stiffness are inseparable considerations for patients. They then lose muscle strength because they are not using the affected limb properly, which affects mobility.” One of the significant impacts of knee arthritis, in particular, they went on, was the loss of function and the associated loss of independence. Even simple things, like getting out of the bath, standing up from sitting, using stairs, or getting out of a car, become tough, adding to a loss of independence, Conaghan explained. “I see people at 45 who have had to give up their physically demanding job and thought they had another 20 years to pay off their mortgage. It is a big deal,” they went on.
This loss of mobility can impact every other element of health, explained Simon. “As you are increasingly in pain and disabled, you move around less. This lack of activity, combined with pain raising the blood pressure, leads to an increased risk for CVD,” Simon said. A whitepaper submitted to the U.S. Food and Drug Administration (FDA) by March et al.3 in 2016 said people living with OA had a pooled prevalence for overall CVD pathology of 38.4% and were almost three times as likely to have heart failure or ischaemic heart disease than those who did not have the condition. It also said that OA significantly limited a person’s ability to self-manage other conditions, such as diabetes and hypertension, and that this, combined with reduced levels of physical activity, was associated with increased all-cause mortality.3
The direct and indirect healthcare system costs associated with OA are also significant.2,3 In 2003 in the USA, total costs attributable to arthritis and other rheumatic conditions were around 128 billion USD, or 1.2% of the country’s gross domestic product for that year. Direct costs from medical expenditure were 80.8 billion USD, while indirect costs from lost earnings were 47 billion USD.3 In the UK, OA is the leading cause of work absence, costing the economy more than 18 billion GBP yearly. Along with other musculoskeletal diseases, it accounts for almost one-tenth of the total annual National Health Service (NHS) budget and 12% of primary care consultations.2 “This is a massive burden on the healthcare system,” said Conaghan.
ARID TREATMENT LANDSCAPE
Despite the considerable impact of OA on individuals, society, and healthcare systems, it remains a significant area of unmet medical need with an inadequate treatment landscape. Conaghan explained that QoL loss derived from the pain and loss of function leads to loss of physical fitness, poor sleep, low mood, and fatigue. “These are important to people, they are a big part of life,” they said. Treatment for these important symptoms, however, is lacking, said Simon: “We have nothing to modify what is considered a progressive, destructive disease.”
There is weak evidence, for example, to support the efficacy of paracetamol or heat or ice packs in OA.4 On oral non-steroidal anti-inflammatory drugs (NSAID), which have been available as an OA treatment option since the 1960s and are recommended in the Osteoarthritis Research Society International (OARSI) guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis,4 Simon said: “These drugs are appealing because they decrease pain and inflammation, but they also carry risk.” The drugs have been associated with an increased risk of heart attacks and stroke, and can result in mucosal injury in the upper, mid-, and lower gastrointestinal tract, which can bring about ulcers, perforation, and bleeding.5 “Probably two-thirds of people cannot tolerate or should not be on anti-inflammatory drugs,” said Conaghan. “Many people experience burning and dyspepsia after they take the drugs, and if you’ve got uncontrolled blood pressure, cardiac disease, or kidney problems, you should not be on an anti-inflammatory.” This is particularly relevant considering the high number of comorbidities among patients with OA.6 While gastrointestinal risks are lower with selective cyclooxygenase-2 inhibitor NSAIDs, they are “not absent,”5 said Simon, adding that while topical NSAIDs are effective and are likely to carry fewer risks, there is currently no evidence regarding their long-term, chronic use. Conaghan also highlighted the practical difficulties associated with using such products. “They probably help, but they may need multiple daily applications, which is not easy for knees if you have trousers or stockings on,” he said.
So-called ‘weak opioids’, such as codeine, are often recommended, despite the low evidence base and high-risk profile. “They constipate you. If you are old, they can make you have falls, and their efficacy is unclear,”7 said Conaghan, adding that if they have a benefit, it was likely only to be short-term. Simon agreed, saying that many patients on opioids become dysphoric and experience falls.7 “If they fall and fracture their hip, one in three will die in the first year,” Simon said.8
Both doctors said there was limited evidence to support hyaluronic acid injections, or viscosupplementation, which aims to reduce pain and increase function by lubricating the joint. A systematic review and meta-analysis published in the British Medical Journal (BMJ) looked at data from >21,163 randomised participants in 169 trials.9 The authors found ‘strong conclusive evidence’ indicating that the approach ‘leads to a small reduction in knee osteoarthritis pain compared with placebo,’ yet the difference was ‘less than the minimal clinically important between-group difference.’ Similar results were observed for function, where sub-analysis found a ‘small, non-clinically relevant improvement’.9
While instant release intra‐articular corticosteroid injections can ease OA symptoms, they are far from an ideal treatment option. “They work, and there is evidence that they work, but only in the short term,”10 said Conaghan. Conaghan also pointed to a 2015 Cochrane review that found the clinically important benefits of corticosteroid injections after 1–6 weeks were unclear.10
In terms of toxicity, every joint injection also comes with infection risk, albeit small.11 In addition, there is an argument that long-term corticosteroid injection use can have a detrimental effect on cartilage integrity.12 However, Conaghan said just one trial, by McAlindon et al.,12 had investigated this question with adequate cartilage measurement and there were limitations in the design. “It used eight injections in a two-year period, which in my experience is more than most patients would get,” they said, adding that the injections were administered independently of pain. “It did show about 50 μm loss of cartilage over two years.12 It was a small loss: is that clinically meaningful? I have no idea, but I suspect not.” Whether corticosteroid use accelerates cartilage destruction is still an open question, said Simon, highlighting a recent head-to-head study that compared intra-articular corticosteroids to hyaluronic acid and found no difference in disease progression.13 Conaghan and Simon agreed the main concern with corticosteroid injections was the short-term benefits, as repeat injections are burdensome for patients and healthcare systems. “We know how to make people feel better when they have an episode of significant pain, swelling, and inflammation,” said Simon. “The problem is how successful is this therapy? For 2–3 weeks after an injection, you feel better, and most people will stay better for months.” However, that is not the case for everyone, meaning more options are still needed.
When all other forms of treatment fail, joint replacement may be the only option. An estimated 33.1% of people with knee OA underwent total knee arthroplasty (TKA) in the USA between 2005 and 2010.14 In the UK, around 90% of >120,000 knee replacements conducted each year are due to OA.15 Simon described the surgical intervention’s results as a ‘miracle,’ but added that it was far from perfect. “Does it have risks? Yes. Is everyone a candidate? No,” Simon said. Considerations include the patient’s age, cardiovascular status, and ability to adhere to post-operative advice. Conaghan said TKA was very effective for people with moderate to severe symptoms, but “it is not straightforward” and “a lot of people do not want a joint replacement. The earlier you have your joint replacement, the more likely you will need revision surgery,” Conaghan said. Around 6% of those who undergo TKA will need revision within 5 years. It is a more technically challenging operation that takes longer than original TKA and is associated with a higher risk of complications, including infection.16 In addition, joint replacement operations are carried out by highly skilled orthopaedic surgeons. As OA prevalence continues to rise, healthcare systems will likely not have the volume of specialist staff needed to meet the corresponding increase in TKA demand, said Conaghan.
They went on to say that preventive, non-pharmacological interventions, including weight loss in patients who were overweight and quadricep strengthening exercises, are extremely important. However, there is often a lack of support for lifestyle-based approaches in primary care and the community, said Conaghan. What’s more, they are most effective when used early in the disease course, and increasing pain, loss of function, and decreased mobility can hinder patients’ efforts to remain active and maintain a healthy weight in the long term.
CHALLENGING PROBLEM
Researchers have attempted to address this unmet medical need for some time, but a lack of knowledge regarding the underlying disease mechanisms has hampered efforts. Simon described OA progression as a heterogenous, intermittent process, adding: “Although everyone talks of this as being a cartilage disease, the origination is very unclear. In the 1970s, it was believed alterations in the subchondral bone meant the cartilage began to bear more transitional forces, leading to degeneration within the cartilage. However, this has never been resolved, and the aetiology is probably multifactorial and different in different people.”
“Whatever the aetiology, relieving pain and maintaining function are among the treatment goals most important to patients,” said Conaghan. “All patient surveys show that these are the things they care about, probably because these are the symptoms their loss of QoL is derived from.” Any new treatment needs to address these endpoints, but the link between structure and symptoms is far from clearcut.
Simon explained: “There are many experimental therapies trying to alter the natural history of the disease, and some people use the term disease-modifying osteoarthritis drugs. But the question is, what does that mean?” A trial of sprifermin, a recombinant human fibroblast growth factor intra-articular injection, published in 2019, demonstrated small regrowth of cartilage.17 “But there was no alteration of symptoms, so we do not know what that means, or the clinical relevance.” Bisphosphonates, which have slowed progression in animal models of OA, are another candidate with disease-modifying potential. However, a 2-year study of 2,483 patients failed to deliver symptom relief despite recording a reduction in C-terminal crosslinking telopeptide of Type II collagen, a marker of cartilage degeneration in progressive OA.18 “Some people got better, but it was not going to treat them all. The same thing happened with metalloproteinase inhibitors,” said Simon. “Some people have tried TNF inhibitors, others have tried IL-1 inhibitors directly into the joint, none of which have worked.”
The challenges to developing new drug products for this patient group are multiple, Simon explained. “It does appear that we might be able to do something for progressive patients, but we do not know how to select them upfront,” said Simon, adding that while work to identify biomarkers is underway, it is still in development. “We are challenged by trial design in the development of drugs and by the heterogeneous nature and progression of the patients,” they said.
A NEW BREED OF STEROIDS?
While research into treatments from biologics to cell therapies continues, an ever-growing number of patients remain in pain and accumulating disability. Finding new ways to use existing, proven medications could be the answer to tackling this unmet need.
“When you look at the evolution of steroids, they are a miracle drug,” said Simon. “Edward Calvin Kendall, Tadeus Reichstein, and Philip Showalter Hench, who invented synthetic steroids, won the Nobel Prize in Physiology or Medicine 1950 for curing rheumatoid arthritis.”19 Simon went on: “These potent anti-inflammatory drugs work, but they have potential side effects over the long term and only offer short-term benefits.”
A new breed of long-acting corticosteroids, however, could tip the risk–benefit profile. Zilretta® (Flexion Therapeutics Inc., Massachusetts, USA), a novel, microsphere-based, extended-release triamcinolone acetonide formulation, has been shown to provide symptom relief for up to 12 weeks.20 However, it is only available in the USA.
EP-104IAR (Eupraxia Pharmaceuticals Inc., Victoria, British Columbia, Canada), a sustained-release fluticasone propionate, is a promising candidate.21 Currently in development, it utilises Eupraxia’s proprietary Particle Release Technology to enable a low early burst of drug release, followed by steady drug release over time (Figure 1). A Phase I, randomised, double-blind placebo-controlled trial of 32 patients found EP-104IAR well tolerated. While the study was not powered to assess efficacy, the authors analysed patient-reported outcome measures to evaluate pain and symptom relief. They found the product provided an immediate improvement in OA symptoms, and these effects persisted for 8–12 weeks.21 If the results can be reproduced in larger trials, Conaghan and Simon said, the product could benefit patients, including those requiring repeat injections.
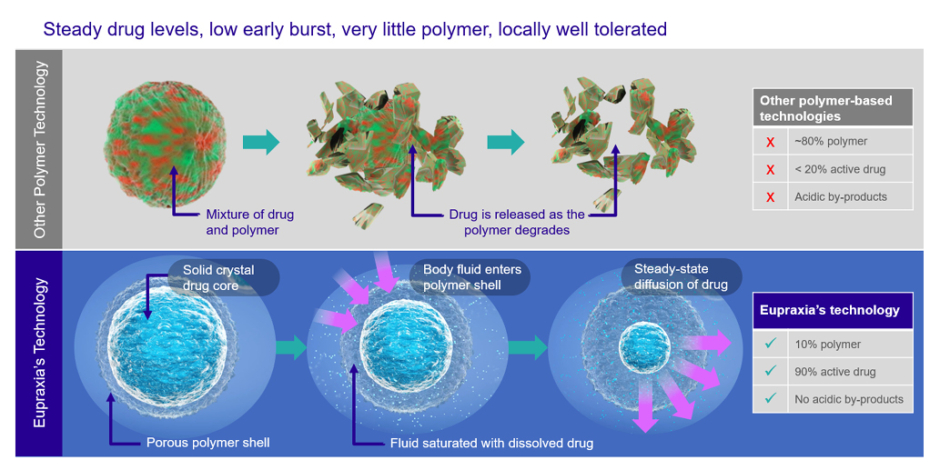
Figure 1: How Eupraxia’s particle release technology works.
“There are clearly patients who will need recurrent injections to alleviate their pain and improve their function,” said Simon. “You want to control their pain and make them more mobile, both of which will improve their blood pressure and decrease cardiovascular risk.” The current debate, Simon said, was how often to administer these injections, with many experts advocating for either more or less frequently than thrice yearly. “All of that has never been resolved, and there are no prospective trials that look at it,” they said. A corticosteroid injection with a 6-month effect, however, would render the question irrelevant. “I think this might be a very important therapeutic to add to our armamentarium,” added Simon.
Conaghan highlighted that this new approach focused on obtaining prolonged benefits from a known, effective therapy. “To be able to leave the drug inside the joint for a longer period addresses two issues. First, if we could give people 6 months of pain relief with a single injection, it could have a lot more effect on their QoL. It would give them the time, function, and ability to work on important things like muscle strengthening.” The second potential benefit is less body exposure to the steroid, Conaghan exclaimed. “What you inject into the joint will get absorbed into the bloodstream. The amount is small, but if you are having repeated injections, it could add up over time.” Slow-release products could have less effect on serum glucose than immediate-release corticosteroid injections, they suggested, benefitting the metabolism of patients with OA in general, and those with comorbid diabetes in particular.22
A longer-acting therapeutic effect, Simon went on, could also potentially help break the cycle of inflammation contributing to progression. “This may be very important because the inflammation may cycle from low grade to high grade, but if you break the cycle, it may be quiescent,” Simon said. “If you alter this cycle, you might change the degeneration of cartilage: it may not regrow cartilage, but it may not worsen.” If this is the case, they went on, it could “change the debate”, but trials are still ongoing.
CONCLUSION
OA is a debilitating, disabling condition that negatively impacts QoL, increases mortality, and places enormous pressure on healthcare systems. Despite OA’s significance and increasing prevalence, the treatment landscape is currently inadequate. There is limited support and resources for non-pharmacological interventions, such as weight loss and muscle strengthening, and the only proven pharmacological treatments, NSAIDs and corticosteroid injections, are far from ideal. In addition, work on disease-modifying osteoarthritis drugs, to date at least, has primarily proved fruitless, leaving a significant area of unmet medical need.
Novel, longer-acting steroids, which may be able to extend the current therapeutic benefit from just weeks to up to 6 months, could be the solution. “If it lasts longer, QoL will be improved, and people will be more mobile. Mobility should give them more control of their blood pressure and more control of their weight, and decreasing weight is associated with decreasing pain in the knees,” said Simon. “Social functioning will be improved, and, for those who need to get recurrent injections, they would not have to keep coming back in to see their doctor and get another needle into their joint.”
References
- Long H et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the Global Burden of Disease study 2019. Arthritis Rheumatol. 2022;74(2):1172-83.
- Conaghan PG et al. Impact and therapy of osteoarthritis: the Arthritis Care OA Nation 2012 survey. Clin Rheumatol. 2015;34(9):1581-8.
- March L et al. Osteoarthritis: a serious disease, submitted to the U.S. Food and Drug Administration. 2016. Available at: https://oarsi.org/sites/default/files/docs/2016/oarsi_white_paper_oa_serious_disease_121416_1.pdf. Last accessed: 2 August 2022.
- Bannuru RR et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578-89.
- Tai FWD, McAlindon ME. Non-steroidal anti-inflammatory drugs and the gastrointestinal tract. Clin Med (Lond). 2021;21(2):131-4.
- Swain S et al. Comorbidities in osteoarthritis: a systematic review and meta-analysis of observational studies. Arthritis Care Res (Hoboken). 2020;72(7):991-1000.
- Fuggle N et al. Safety of opioids in osteoarthritis: outcomes of a systematic review and meta-analysis. Drugs Aging. 2019;36:129-43.
- National Institute for Health and Clinical Excellence (NICE). Hip fracture: the management of hip fracture in adults. 2009. Available at: https://www.nice.org.uk/guidance/cg124/documents/hip-fracture-final-scope2#:~:text=b)%20Mortality%20is%20high%20%E2%80%93%20about,are%20attributable%20to%20the%20fracture. Last accessed: 2 August 2022.
- Pereira T et al. Viscosupplementation for knee osteoarthritis: systematic review and meta-analysis. BMJ. 2022;378:e069722.
- Jüni P et al. Intra‐articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;2015(10):CD005328.
- Kijowski R. Risks and benefits of intra-articular corticosteroid injection for treatment of osteoarthritis: what radiologists and patients need to know. Radiology. 2019;293(3):664-5.
- McAlindon TE et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317(19):1967-75.
- Bucci J et al. Progression of knee osteoarthritis with use of intraarticular glucocorticoids versus hyaluronic acid. Arthritis Rheumatol. 2021;74(2):223-6.
- Ward MM. Osteoarthritis care and risk of total knee arthroplasty among Medicare beneficiaries: a population-based study of regional covariation. Arthritis Rheumatol. 2021;73(12):2261-70.
- Versus Arthritis. New procedure for early osteoarthritis to reduce need for knee replacement. Available at: https://www.versusarthritis.org/research/research-achievements/new-procedure-for-early-osteoarthritis/. Last accessed: 2 August 2022.
- Lee DH et al. Causes and clinical outcomes of revision total knee arthroplasty. Knee Surg Relat Res. 2017;29(2):104-9.
- Hochberg MC et at. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: the FORWARD randomized clinical trial. JAMA. 2019;322(14):1360-70.
- Bingham CO et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 2006;52(11):3494-507.
- The Nobel Prize. The Nobel Prize in Physiology or Medicine 1950. 1950. Available at: https://www.nobelprize.org/prizes/medicine/1950/summary/. Last accessed: 2 August 2022.
- Conaghan P et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Joint Surg Am. 2018;100(8):666-77.
- Malone A et al. Safety and pharmacokinetics of EP-104IAR (sustained-release fluticasone propionate) in knee osteoarthritis: a randomized, double-blind, placebo-controlled phase 1 trial. Osteoarthr Cartil Open. 2021;3(4):100213.
- Russell SJ et al. Triamcinolone acetonide extended release in patients with osteoarthritis and type 2 diabetes: a randomized, phase 2 study. Rheumatology (Oxford). 2018;57(12);2235-41.








