Meeting summary
Epilepsy is a common neurological condition that results from abnormal electrical activity in the brain and is characterised by recurrent seizures. Perampanel is a first-in-class, non-competitive antagonist of the ionotropic α-amino-3-hydroxy-5- methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor on post-synaptic neurons, which is licensed for the treatment of certain types of epilepsy. In this interview, an expert neurologist reviewed evidence from a post-hoc analysis of the PERMIT study, which evaluated the efficacy and safety of perampanel used as early versus late add-on therapy for people with epilepsy with focal and generalised seizures.
INTRODUCTION
Perampanel is indicated for the adjunctive treatment of focal onset seizures with or without secondarily generalised seizures in patients from 4 years of age, and primary generalised tonic-clonic seizures in patients from 7 years of age with idiopathic generalised epilepsy.1 The PERMIT study was a pooled global analysis that evaluated the use of perampanel in routine clinical practice to treat patients with focal and generalised epilepsy.2,3 The following article focuses on results from an on-licence post-hoc analysis of the PERMIT study, which evaluated the effectiveness, safety, and tolerability of perampanel when used as early versus late add-on therapy.2 During an interview conducted by the European Medical Journal (EMJ), Vicente Villanueva, Neurologist and Head of the Refractory Epilepsy Unit at the Hospital La Fe de Valencia, Spain, considered the clinical practice implications of these real-world data and highlighted how results support the use of perampanel as a broad-spectrum, early add-on therapy to treat focal and generalised seizures.
BACKGROUND TO THE PERMIT STUDY
The PERaMpanel pooled analysIs of effecTiveness and tolerability (PERMIT) study was a pooled analysis of data from 44 real-world studies/work groups conducted across 17 countries, in which people with focal and generalised epilepsy were treated with perampenel.3 PERMIT represents the largest perampanel real-world epilepsy study ever conducted, and the corresponding dataset now includes nine published papers, with a further two manuscripts in progress.2-10
Asked about the size and robustness of the PERMIT dataset, Villanueva explained that the study was important due to its large scale and broad patient population, which reflects that seen in daily clinical practice. All these patients were part of real-world, prospective, retrospective, and cross-sectional studies conducted around the world. More than 5,000 patients from five continents with focal and generalised epilepsy were included, all of them treated with perampanel in everyday clinical practice, he elaborated. The large size of the PERMIT cohort has also enabled meaningful subgroup analyses to be carried out.2
EVIDENCE FROM REAL-WORLD POST-HOC ANALYSIS
The current post-hoc analysis of the PERMIT study explored outcomes in patients treated with perampanel according to its summary of product characteristics as add-on therapy.2 As Villanueva explained, patients in the early add-on group were those in whom perampanel was used after the failure of one or two anti-seizure medications (ASM). This group was then compared to patients with late add-on, in whom perampanel was used after the failure of three or more prior ASMs. “We analysed retention, efficacy, safety, tolerability, and dosage at time points of 3 months, 6 months, and 1 year; we also set a final visit,” he noted.
Villanueva detailed how the PERMIT full analysis set included 5,200 patients with epilepsy (PWE), “and from those, 4,045 patients were treated with perampanel as add-on therapy, 1,184 patients used perampanel as early add-on, and 2,861 patients were using perampanel as late add-on therapy.”2 In terms of baseline characteristics, Villanueva pointed out that patients in the latter group had a longer mean epilepsy duration, a higher number of previous and concomitant ASMs, and more intellectual disability and psychiatric comorbidities, which he described as “typical of this late add-on setting”.
“Something very important, particularly in terms of safety,” Villanueva emphasised, “is that in the early add-on group, the mean perampanel dosage [at the last visit] was 5.7 mg [per day]. However, in the late add-on [group] it was 6.7 mg per day. This is something that is remarkable and is a good way to explain the difference in terms of tolerability.”
EFFICACY AND SAFETY OUTCOMES
In this post-hoc analysis, effectiveness was evaluated by seizure type and included assessments of seizure freedom rate and responder rate.2 Seizure freedom rates with perampanel were significantly higher in the early as compared to the late add-on subgroup at all time points for total and focal seizures, while seizure freedom from generalised tonic-clonic seizures was significantly higher at the last visit for the early add-on patients (Figure 1).2 As Villanueva explained, when the median reduction in monthly seizure frequency was analysed from baseline to last visit, there was a statistically significant difference in terms of total seizures; 93% median seizure reduction for the early add-on versus 68% for the late add-on. Similarly, with focal seizures, the reduction was 80% for the early add-on group and 47% for the late add-on group. “That means that in the earlier setting, outcomes were better,” he remarked.
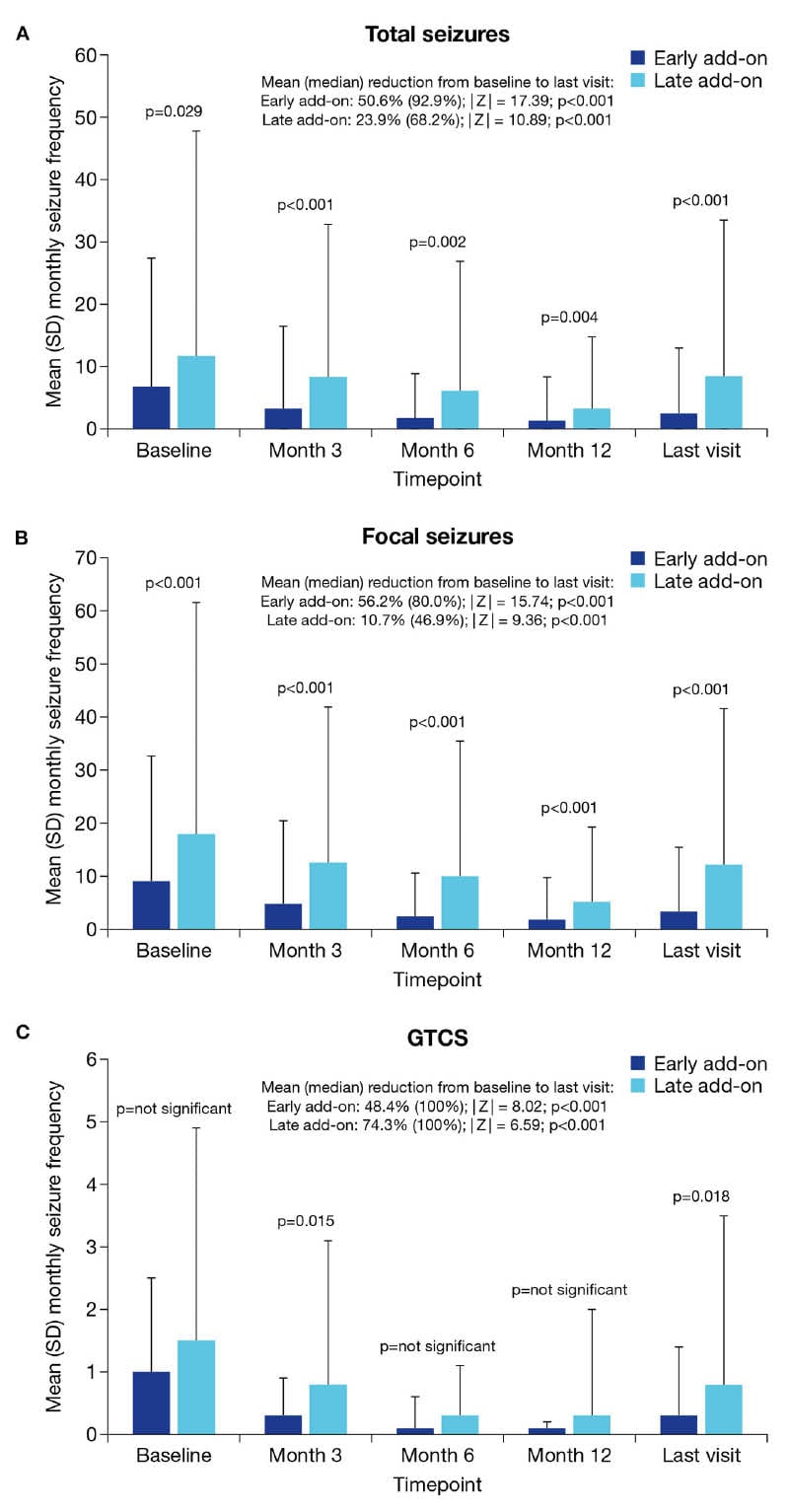
Figure 1: Mean monthly seizure frequency at baseline, Month 3, Month 6, Month 12, and the last visit in the early and late add-on subgroups.2
A) Total seizures. B) Focal seizures. C) GTCS.
GTCS: generalised tonic-clonic seizures; SD: standard deviation.
“However, when we analysed generalised tonic-clonic seizure [the reduction] was 100% in both groups. So, in the group with generalised tonic-clonic seizures, even in late stages of treatment, outcomes with perampanel are still quite high,” he added.
Responder rates, defined as a ≥50% reduction in seizure frequency from baseline with perampanel treatment, were also significantly higher in the early versus late add-on subgroups at all timepoints for total and focal seizures, and at Month 12 and the last visit for generalised tonic-clonic seizures.2
Considering perampanel retention rates, Villanueva explained that these were not statistically significantly different at Month 3 (~90%) or Month 6 (~80%) for the early versus late add-on groups. However, when the difference in retention rate at the end of Month 12 was analysed, this was 67% in the early add-on setting versus 62% in the late add-on setting, which is statistically significant. “So, it seems, in the long term, the retention was better in the early add-on compared to the late add-on group,” he remarked (Figure 2).
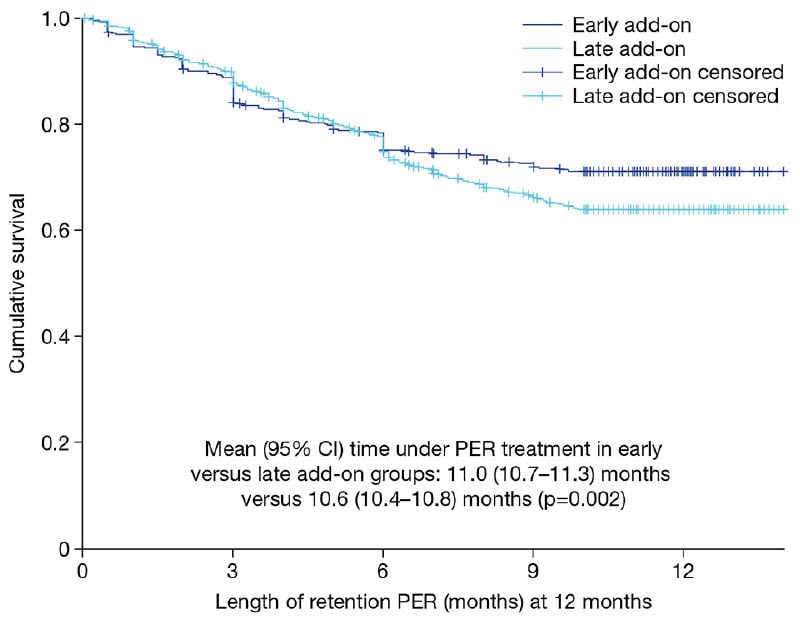
Figure 2: Kaplan-Meier curve for retention on perampanel treatment over 12 months in the early and late add-on subgroups.2
PER: perampanel
Regarding the safety profile, Villanueva reported that: “The percentage of adverse events was lower in the early add-on versus late add-on groups, and this was statistically significant, 42% in the early add-on versus 54% in the late add-on. When we analysed those patients with any type of adverse event that led to discontinuation, again, this was statistically significantly better in the early add-on versus the late add-on groups, 15% versus 18%.” Villanueva also noted that the types of adverse events were “quite similar” in both groups, most commonly dizziness, vertigo, somnolence, and irritability, and there was no significant difference in the proportion of patients experiencing psychiatric adverse events (Table 1).
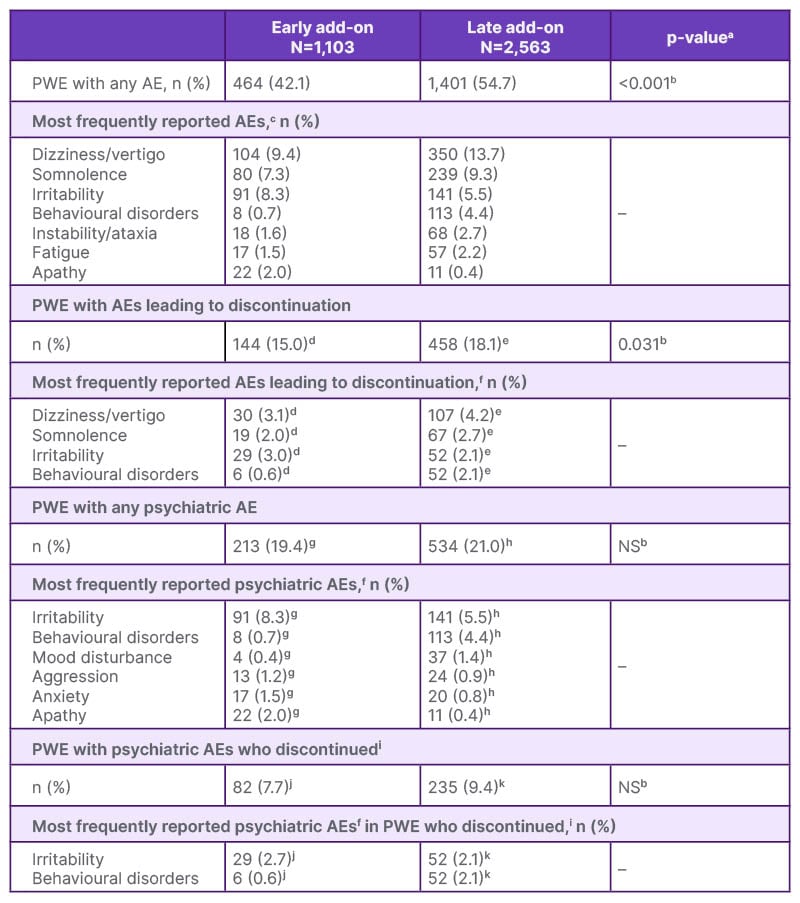
Table 1: Summary of safety and tolerability.2
aFor comparisons between the early vs. late add-on groups.
bChi-squared test.
c≥2% of PWE in either group.
dN=957. eN=2,525.
f≥1% of PWE in either group.
gN=1,099.
hN=2,543.
iThese PWE had psychiatric AEs but it was not possible to determine if it was these AEs that led to discontinuation.
jN=1,066.
kN=2,491.
AE: adverse event; NS: not significant; PWE: people with epilepsy.
As to the reason why perampanel was significantly better tolerated in the early add-on setting, Villaneueva postulated that “those patients in the late add-on were treated with more concomitant ASMs, [which] could contribute to more adverse events.”
“The dosage is also important,” he reiterated, “the lower dosage used in the early add-on setting is enough to treat [these patients] and could reduce the percentage of adverse events and those that led to discontinuation.”
CLINICAL PRACTICE IMPLICATIONS
Villanueva stressed that findings from this post-hoc analysis provide a reliable evidence base from which to draw conclusions about the potential benefits of early perampanel initiation, because outcomes came not only from one group of patients or one country, but from different countries where different clinics were using this medication.
“I think this study is important for clinical practice or for those that are dealing every day with patients with epilepsy because it conveys some practical, important messages,” he elaborated. “Perampanel was significantly more effective when used in the early add-on versus late add-on [setting] to treat patients with focal and generalised epilepsy. The incidence of adverse events and the rate of discontinuation due to adverse events were also significantly lower in patients treated in the early add-on setting versus late add-on therapy.”
On the interpretation and application of these findings in real-world practice, Villanueva raised three important points. “First of all, perampanel is a broad-spectrum medication that is useful in focal and generalised [tonic-clonic] seizures. This is important because sometimes, in the early add-on setting particularly, it is not so clear for some patients what type of epilepsy they have,” he explained. “Second, the dosage that is used in the early add-on setting is lower, and this is good because tolerability is going to be better. And third is that, based on these good outcome data in the early add-on setting, we have support in practice to start using this medication in those patients that have failed one or two ASMs.” He added that, although these data support the early use of perampanel, efficacy is also retained in later stages of treatment.
Finally, Villanueva shared two patient case studies from his own clinical practice, illustrating the use of perampanel as first add-on therapy. The first was a female patient in her 40s who initially presented with focal seizures and was successfully treated with lacosamide, dose-titrated up to 400 mg a day. However, her seizures subsequently evolved into bilateral tonic-clonic seizures, occurring predominately during the night, which led to a significant negative impact on her quality of life. Villanueva explained that this patient was started on perampanel as an early, first add-on therapy, and the dosage increased progressively to 8 mg. “Now, with the dosage of 400 mg lacosamide and 8 mg of perampanel, she has been seizure-free for maybe 3 or 4 years, and her life has totally changed, it’s back to a normal life without issues,” he remarked.
The second case was an 18-year-old male patient with idiopathic generalised epilepsy who was initially treated with levetiracetam. As Villanueva outlined, “We increased the dosage [of levetiracetam] but were not able to completely eliminate seizures, then we decided to start perampanel in this patient with generalised tonic-clonic seizures within a primary idiopathic generalised epilepsy. We increased the perampanel dosage progressively to 6 mg a day, and after reaching this dosage, the seizures disappeared. With this combination, this patient has now been seizure-free for years.” Villanueva also noted that the ability to take perampanel at bedtime had helped to improve this patient’s compliance with treatment.
Please note that post-hoc analyses are not powered to detect a difference, and clinically relevant decisions should not be drawn from these.
CONCLUSION
Overall, this post-hoc analysis of the PERMIT study demonstrated that perampanel was effective and generally well-tolerated when used as early or late add-on therapy but was significantly more effective and better tolerated when initiated early in the treatment journey. Villanueva concluded that these results “support the use of perampanel as early add-on therapy in patients with focal and generalised seizures, based on the good outcomes we observe in this population of patients.”
EMEA-FYC-24-00074
May 2025







