Abstract
The medical management of women with epilepsy is an evolving specialization within neurology and epileptology. This article highlights the major topics and key challenges, providing clinical insights regarding best current practices alongside associated clinical rationales and recommendations within this emerging field. Additionally, this article identifies opportunities for further study and outlines current gaps and limitations in understanding.
Key Points
1. The comprehensive evaluation and management of women with epilepsy is an evolving specialization within neurology and epileptology that clinically assesses issues that impact the entire reproductive lifespan. Such adjunctive management assists patients planning for and going through pregnancy in collaboration with other healthcare providers.
2. This article highlights major clinical topics and summarizes the current understanding of best practices and recommendations relevant to specialized consultations. While it emphasizes pregnancy related care, it also covers other issues that affect patients throughout their lifespan.
3. Several important issues remain unresolved, and this article outlines these gaps, emphasizing the need for further research to optimize the adjunctive role of emerging therapies and future developments.
INTRODUCTION AND OVERVIEW OF SEIZURES
This work highlights seizure disorders and summarizes the major topics, key challenges, and clinical insights into how seizure disorders impact women across the reproductive lifespan. It highlights and summarizes the current understandings of many topics such as hormonal influences on the menstrual cycle, catamenial epilepsy (CE), and the impact of seizure disorders on contraception and fertility, alongside issues surrounding bone health and menopause in patients with seizure disorders and sexual dysfunction, and the current understanding of genetic and inheritance risks of seizure disorders. Additionally, the article highlights the evolving practice of pre-pregnancy planning and issues surrounding pregnancy, including anti-seizure medication (ASM) management, teratogenicity, and postpartum care, including breastfeeding and ASM tapering. This review notes some issues affecting males, concluding with an overview of some potential future directions, gaps in the literature, and where further study might be indicated.
WHAT IS A SEIZURE?
A seizure is a pathological disturbance of the normal or baseline electrocorticography or electrical rhythm of the brain that may result in abnormal clinical manifestations, although some seizures may be subclinical without readily observable signs.1-4 The location of the seizure discharges may cause motor signs and clinical manifestations, depending on what cortico-functional anatomy or networking is activated, and signs and symptoms depend on the particular anatomical localization, spread, and termination of such abnormal electrical activities within specific brain regions.5,6 Seizures that occur in response to provocative factors, for example, metabolic derangements, may not be indicative of epilepsy, which refers to a condition where there are continuous risks of unprovoked seizures. In contrast, a seizure occurring in the appropriate physiological context may be expected and not pathological, whereas epilepsy involves a persistently increased probability of seizures.1-6 Seizures, and especially uncontrolled or repetitive seizures, may result from and alter brain function and cortical functional anatomy over time, potentially triggering seizures in other brain regions through a process known as kindling.7-10 Seizure disorders can significantly impact neurocognition and quality of life, both due to the seizures themselves and associated treatments.11 Seizures may be associated with injury, loss of independence, and decreased self-esteem. They have also been linked to mood disorders, sudden unexplained death in epilepsy, and other injuries, especially when poorly controlled.12,13 The major etiologies of seizures include idiopathic ones, or unknown origins, which account for approximately two-thirds of cases. Other etiologies of epilepsy include syndromes such as mesial temporal sclerosis, head trauma, intracranial tumors, strokes, hemorrhages, neurovascular lesions, neuronal migration disorders, metabolic abnormalities, genetic causes, and any other condition that disrupts functional cortical anatomy, thereby chronically lowering the seizure threshold.14-20
How the relevant issues concerning women and the reproductive lifespan impact and influence the occurrence of seizures and vice versa will be reviewed within the framework of the various subtopics that follow in this review.
HORMONAL INFLUENCES AND THE MENSTRUAL CYCLE
Both estrogen and progesterone levels exert hormonal control on the menstrual cycle, and the relative balance of estrogen to progesterone is considered the key factor.21 Ovulation is caused by a relative surge in estrogen, and for menstruation to occur, this surge must be followed by progesterone levels falling lower than those of estrogen.21 During anovulatory cycles, in which more seizures might occur, estrogen levels remain relatively high compared to levels of progesterone.21-23 Higher estrogen or an increased estrogen-to-progesterone (E/P) ratio can potentiate glutamate receptors, leading to increased neuronal excitability and a higher risk of seizures. In contrast, a higher progesterone-to-estrogen ratio may facilitate gamma-aminobutyric acid (GABA) and chloride receptors, which have inhibitory effects on seizure activity.22,23
Estrogens are thought to be epileptogenic through several mechanisms, including those noted above, as well as their effects on the cycling of GABAA and GABAB receptors.21-23 Estrogen and progesterone ratios are also linked to effects mediated by calcium and chloride influx at GABAA receptors. These hormones modulate hippocampal glutamate receptors with estrogens acting as agonists, while estrogens may also increase both dendritic arborization and sprouting, and excitability in such neuronal networks, causing the CA1 region of the hippocampus to be more susceptible to glutamate-induced excitation.21-23 Progesterone is generally thought to be protective, as noted above, with promotion of enhanced synthesis of GABAA and GABAB receptors promoting increased chloride influx, potentially reducing glutaminergic activity.21-23
During puberty and menarche, it is common to experience the onset or worsening of seizures due to a relatively increased E/P ratio. Hormone replacement may have corresponding effects, generally increasing seizure frequency when the E/P ratio is high, and decreasing seizures when the ratio is lower, according to the literature.21-23
Self-reported sexual dysfunction is a common complaint, as approximately 40% of people with epilepsy report complaints including decreased sexual desire, increased dysmenorrhea, increased anxiety and impaired social functioning, and negative effects on self-esteem.23 These issues are related to seizure frequency, drug exposure, central nervous system effects or dysfunction, and altered impacts on central molecular substrates such as serotonin and prolactin. The levels of these substrates may be reduced and influenced by ASMs.22-24
Birth rates in general are reduced in women with epilepsy due to an increased incidence of irregular menses and anovulatory cycles. An estimated 25–40% of menstrual cycles in women with epilepsy may be anovulatory, compared to about 10% in women without epilepsy.22-24 Additionally, there is an increased incidence of polycystic ovaries and reproductive endocrine disorders in women with epilepsy, with some effects potentially due to the ASMs. For example, enzyme-inducing ASMs might reduce estradiol, have effects on androgens, and contribute to complex effects on steroid metabolism.22-24 Polycystic ovaries have been noted in approximately 15% of women without epilepsy, but occur in approximately 30% of women with epilepsy, especially those who are taking /valproate. 22-25 However, not all women with polycystic ovaries exhibit polycystic ovarian syndrome, a condition associated with an abnormality in insulin receptors leading to insulin resistance, obesity, acne, hirsutism, elevated luteinizing hormone, abnormal effects on androgens, abnormal lipids, and anovulation.22-25 The consequences of polycystic ovarian syndrome include infertility, dyslipidemia, diabetes mellitus, and endometrial cancer.25
In rare cases, hormonal effects, predominantly estrogen or progesterone ratio increases, might enhance the growth of an arteriovenous malformation or make such lesions symptomatic due to growth mediated at the receptor level. This stems from impacts on the E/P ratios, angiogenesis or blood flow effects, including volume of distribution, and renal and liver metabolism of these hormones.22-26
A Brief Summation of Hormonal Influences on the Occurrence of Seizures
- Estrogen or increased E/P ratio: increased seizures due to activation of glutaminergic mechanisms, which are excitatory.
- Progesterone or decreased E/P ratio: decreased seizures due to the modulation of GABA receptors, which are inhibitory.
- Estrogen has effects on the promotion of kindling, impacting the modulation of hippocampal CA1 pyramidal cells and increasing sensitivity to excitatory input.
A Brief Summation of Seizure Disorders and Sexual Functioning
- Decreased sexual desire: increased dysmenorrhea with anovulatory cycles and impact on gonadotropin-releasing hormone (GnRH) and association with early menopause.
- Increased anxiety.
- Impaired social functioning with negative effects on self-esteem.
- Association with polycystic ovary syndrome.
- Lower birth rate in males, as well as decreased libido and testosterone, and impaired spermatozoa; however, this could be due to confounding issues of medications.
CATAMENIAL EPILEPSY AND HORMONAL TREATMENT STRATEGIES FOR EPILEPSY IN WOMEN
CE occurs in up to approximately 70% of women with epilepsy and is defined in the current literature as seizures that occur at up to twice the baseline frequency during specific phases of the menstrual cycle. Due to the previously noted hormonal influences, CE is thought to result from elevated estrogen levels or an increased E/P ratio, which promote glutamatergic excitation, facilitate kindling, and lower the seizure threshold. In contrast, progesterone has anticonvulsant properties, enhancing allosteric modulation of GABA receptors.21,22,27-29 In patients with CE, there may be either regular or irregular menstrual cycles, and seizures may occur around ovulation, just prior to menses or within approximately 3 days before to 3 days after ovulation. There is a significant association with anovulatory cycles (approximately 35% of patients with epilepsy as opposed to about 8% of controls).27-31 Stress, sleep deprivation, and reduced serum concentration of ASMs from non-compliance may enhance the occurrence of catamenial seizures.27-31 The mainstay of treatment of patients with irregular menses is intramuscular (IM) medroxyprogesterone or oral contraceptive pills (OCP) with withdrawal weeks.27-31 Seizures in women with epilepsy typically occur just before or during menstruation, when there is an increase in the E/P ratio or a drop in progesterone. Seizures are also more likely to occur mid-cycle during ovulation, when estrogen levels surge. In anovulatory cycles, seizures tend to occur more frequently during the second half of the menstrual cycle.27-31
Progesterone can be used as a mainstay of treatment, particularly in patients with catamenial seizures and regular menstrual cycles. It is typically administered as 100–200 mg lozenges or capsules up to three times a day during Days 14–25 of the menstrual cycle, with withdrawal occurring on Days 26–28. This regimen targets the luteal phase of the menstrual cycle, which includes the peri-ovulatory period.27 Side effects may include weight gain, acne, breast tenderness, irritability, and weight gain, and such treatment may induce perimenopause or menopause in some patients.27-31
The most specific treatment for patients is based on identifying the specific patterns of seizure occurrences that might occur with the menstrual cycle. Herzog et al.29 proposed three designations, including a C1 pattern, which is perimenstrual, C2, periovulatory, and C3, which is associated with an inadequate luteal phase. The C1 pattern involves seizures that occur from Days 0–3 of the menstrual cycle, prior to the ovarian follicular phase.29-31 The C2 pattern of CE involves seizures that occur from approximately Days 10–14, or the pre- or periovulatory phase, of the menstrual cycle.29-31 The C3 pattern occurs subsequently to the C2 designation at the latter stages of the periovulatory phase, and extends through the luteal phase and menstrual phases, while the C3 pattern is highly associated with anovulation.29-31 Treatment of patients with regular cycles includes natural progesterone lozenges during the luteal phase (from approximately Days 14–25).29-31 The treatment protocols for patients with irregular menses (C2 and C3 patterns, or failed treatment with progesterone lozenges during the luteal phase of the C1 type) include increasing baseline ASMs for several days, using or adding benzodiazepines, clobazam, or implementing acetazolamide for a number of days during the menstrual cycle.29-31
CONTRACEPTION AND FERTILITY
ASMs may be affected by OCPs and vice versa.27-31 The effectiveness of the OCPs may be significantly reduced, especially those that are metabolized by the cytochrome p450 system and impacted by ASMs.27-31ASMs may cause reduced binding of OCPs to receptors and may enhance metabolism of OCPs, making the OCPs less effective.27-31 Subdermal or progesterone levels may fall due to enhanced metabolism, which can result in mid-cycle spotting and an increased risk of pregnancy. Low-dose OCPs containing approximately 30–33 mcg of estrogen, with around 50 mcg of estrogen, may help mitigate these effects.27-31 Knowledge about such interactions is critical for the optimal management of these patients.
Current standards indicate that for patients on enzyme-inducing agents, the first line of contraception would be depot medroxyprogesterone acetate 150 mg, administered with a dosing interval of 10–12 weeks. The second-line option would be an intrauterine device that is either copper-based or progestin-based.27-31 It is noteworthy that for emergency contraception, levonorgestrel might require a second dose due to metabolic effects from the enzyme-inducing ASMs.27-31
For patients not taking enzyme- inducing agents, there are no absolute restrictions on OCPs; however, medications should be monitored for potential serum changes. For example, lamotrigine may require dose adjustments when initiating or terminating treatments.
PRE-PREGNANCY PLANNING AND CONSULTATION
Pre-pregnancy planning and consultation, with and having an expert input focused on epilepsy and the female reproductive lifespan, is an evolving field. It is becoming increasingly available in epilepsy centers as a comprehensive way to ascertain and manage medical care for seizure disorders, particularly in relation to an upcoming pregnancy.11,32-35 Recent literature and studies, such as the WEPOD study by French et al.,36 indicate that female patients with epilepsy seeking pregnancy had a comparable likelihood of achieving pregnancy, time to achieve pregnancy, and pregnancy outcomes compared to a group of healthy controls, when their care needs are addressed. These findings should reassure both patients and clinicians during counseling for these women who are planning a pregnancy.36
Preconception management of pregnancy in women with epilepsy involves counseling, establishing baseline seizure frequency, and determining the necessary ASM blood levels. It also includes assessing whether monotherapy or polytherapy is indicated. Folic acid supplementation, up to 4 mg/day, is encouraged, and counseling should caution patients against the abrupt withdrawal of ASMs, which could be associated with a significant amount of breakthrough seizures (occurring in approximately 50% of patients). In general, ASMs should be prescribed at the lowest possible dose and thereby minimize the teratogenic risk.31-35 Compared with monotherapy, polytherapy increases risk of intrauterine death, cesarean section, low birth weight, premature birth, risk of poor neural development, and major malformations.31-35 Folate should be started according to recent practice guidelines within 3 months of pregnancy to reduce the risk of neural tube defects and to improve IQ and neurodevelopmental outcomes, as some research has indicated that folate might reduce autistic traits.31-35,37-43 While the American College of Obstetrics and Gynecology (ACOG) guidelines include recommending high-dose folate up to 4 mg/day starting during the month prior to conception, the UK and European guidelines are considering recommending up to 5 mg/day.37-43
ASMs and their relevant side effects, with a focus on women with epilepsy, should be highlighted. Phenytoin is associated with dysmorphisms and coarsening of features with chronic use, and significant weight gain is associated with valproic acid and carbamazepine.6,28,31-35,41-42 Weight loss is associated with zonisamide and topiramate, while enzyme-inducing ASMs, especially valproic acid, might contribute to polycystic ovaries as well as hair thinning, or hirsutism, depending on the specific metabolism.6,28,41,42
MANAGEMENT OF SEIZURES DURING PREGNANCY: AN OVERVIEW OF CURRENT PRACTICE STANDARDS
Anti-Seizure Medication Management, Teratogenicity, and Complications
As previously mentioned, effective seizure management during pregnancy is essential for optimal outcomes in this evolving specialization. ASM monitoring should be encouraged every 4 weeks during pregnancy. Baseline doses may need to be increased by approximately 35% from preconception levels, whether based on dosage or serum levels, due to physiological changes such as enhanced hepatic metabolism, increased renal blood flow and clearance, altered protein binding, and increased volume of distribution. These changes generally lead to decreased serum levels of ASMs as pregnancy progresses.31-35 ASM monitoring should be done more frequently, weekly if possible, during the last trimester and final month of pregnancy, as seizures, particularly generalized tonic–clonic seizures (GTCS), are associated with placental abruption and significant utero-placental insufficiency. These events could lead to potentially significant effects such as hypoxemia or hypoperfusion, potentially resulting in fetal organ damage.31-35 Ultrasonography to identify neural tube defects, such as anencephaly and myelomeningocele, may be performed at Week 13 of gestational age.31-35,43-46 Orofacial clefts and cardiac defects are detectable at approximately 20 weeks. The literature does not currently recommend any preferred methods of delivery, such as an electively planned cesarean section as opposed to a normal and spontaneous vaginal delivery.31-35,43-45 Vitamin K should be given for the final month of pregnancy if hepatic-inducing ASMs are being used.31-35,43-47
Observations and older literature, largely unchanged since the 1980s, indicated that newborns of mothers treated with enzyme-inducing ASMs are at risk of hemorrhagic disease of the newborn. This is likely due to increased turnover of vitamin K due to hepatic enzyme induction affecting the vitamin K-dependent clotting factors II, VII, IX, and X.46,47 A prior, well-known, pivotal study in1985 by Motohara K et al.,46 identified cohorts of babies born given vitamin K compared to those that were untreated with vitamin K. The untreated group had a higher number of PIVKA II-positive babies, particularly those born to mothers on enzyme-inducing ASMs, in a study of fetal cord blood, supporting the idea that vitamin K deficiency was a factor.47 The study by Motohara K et al.46 indicated that the risks of hemorrhagic complications in newborns could be predicted by PIVKA II proteins as a biomarker. The presence of these proteins supports the recommendation of administering 10 mg of vitamin K daily during the last month of pregnancy for women taking enzyme-inducing ASMs, which is effective in preventing hemorrhagic disease of the newborn.47
Practice Guidelines: Women with Epilepsy
- Pre-pregnancy planning is recommended to establish a risk assessment, determine whether monotherapy or polytherapy is indicated, include baseline ASM levels, and note dosing for the overall strategy to prevent GTCS, with monthly levels monitored until the last trimester/month, when levels might be done weekly or biweekly.
- Folate 1 mg/day for women with epilepsy generally, but 4 mg/day within 3 months of conception.
- European Countries and UK recommend 5 mg/day.
- Vitamin K supplementation during the last month of pregnancy to prevent hemorrhagic disease of the newborn for mothers taking enzyme-inducing ASMs.
- Breastfeeding should be generally encouraged.
- The risk of major fetal malformations are highest with valproate.
- Neural Tube defects may be screened at 13 weeks (for anencephaly and myelomeningocele) and 20 weeks (for orofacial clefts and cardiac defects) with high-level ultrasounds.
- Consideration of dose adjustment of medications during pregnancy, due to changes in volume of distribution, hepatic and renal clearance, and metabolism, and after post-partum to prevent toxicity, with lamotrigine requiring rapid taper of a few days and most other ASMs within 3 weeks.
- Consider bone health screening and calcium and vitamin D supplementation in women with epilepsy who are on enzyme-inducing ASMs, with calcium 1000–1500 mg/day and vitamin D 400–800 IU/day.
ANTI-SEIZURE MEDICATIONS AND NEUROCOGNITIVE RISKS IN OFFSPRING
Current Practice and Evolving Literature
Seizure medications are associated with major congenital malformations, dysmorphism, preterm birth, intrauterine growth retardation, and potentially neurocognitive and neurodevelopmental adverse effects.32-43
The NEAD study enrolled pregnant women with epilepsy who were taking a single antiepileptic agent (carbamazepine, lamotrigine, phenytoin, or valproate) in a prospective, observational, multicenter study in the USA and the UK to determine whether, and the extent to which, ASMs had neurocognitive effects.48 The primary analysis reviewed neurodevelopmental outcomes at 6 years of age, after exposure to different antiepileptic drugs in utero. The report focused on a planned interim analysis of cognitive outcomes in 309 children at 3 years of age and noted that in utero exposure to valproate, compared to other commonly used antiepileptic drugs, was associated with an increased risk of impaired cognitive function at 3 years of age.48 The 2013 publication regarding the NEAD study, by Meador et al.,49 also identified that fetal valproate exposure had dose-dependent associations with reduced cognitive abilities across a range of domains at 6 years of age. Additionally, reduced right-handedness and verbal (versus non-verbal) abilities may be attributed to changes in cerebral lateralization induced by exposure to antiepileptic drugs. The positive association of periconceptional folate with IQ was consistent with other studies.49 Additional reports, such as in both a follow-up 2019 and 2020 analysis, illustrate further benefits of folate with improved neurocognitive outcomes when combined with ASM exposure.50,51
POST-PARTUM ISSUES AND BREASTFEEDING
Generally, ASM concentration in breast milk is about one-third or less of that in serum, and is further reduced depending on the drug’s protein binding. These levels are significantly lower than those seen with in utero exposure.52,53 The author suggests that if breast milk is relatively safe, the presence of ASMs may not significantly influence organogenesis, as that process is already complete by the time breastfeeding is considered or initiated.52,53 While there may be significant benefits to maternal–fetal bonding and the development of appropriate immune mechanisms, and while literature cited above notes benefits of breastfeeding in women with epilepsy, poor feeding, irritability, or lethargy may indicate that such an infant might be intolerant of the ASMs.52,53 Drugs with high protein binding, such as phenytoin, carbamazepine and derived compounds, valproic acid, and other enzyme inducers might offer lower levels in breast milk compared to medications with low protein binding. This may lead to relatively higher levels in breast milk for drugs such as levetiracetam, gabapentin, ethosuximide, topiramate, and zonisamide.42-45
Generally, most ASMs are classified as pregnancy category C or worse, primarily due to their association with neural tube defects.42-45 Of note, there may not be exhaustive studies in humans, and such a categorization indicates that some pregnant animals treated with such medications had babies with medical issues or malformations, although the medication might still be helpful and a risk–benefit analysis would be required for use and full recommendations in clinical settings.44,45
Tapering of ASMs should generally occur within a few weeks of delivery as maternal physiologyreturns to baseline pre-pregnancy status; however, due to stressors and sleep deprivation, some relatively higher dosing than baseline might be considered (approximately at 125% of baseline values).33-35 Strategies to allow for sleep and stress mitigation should be sought to minimize the risk of seizures, with encouragement of a good diet, hygiene, and a survey for mood changes and post-partum depression.4,14,28,33-35,44,45 For some medications, maternal metabolic changes after delivery, such as reduced drug clearance due to the absence of fetal liver and kidney function, and a decreased volume of distribution, may warrant tapering the dose by 50% within 3 days postpartum, with lamotrigine, in particular, often requiring the most rapid tapering.11,28,33-35,43-45
Current literature has indicated that, generally, breastfeeding should be encouraged regardless of ASM exposure, as breastfeeding facilitates maternal–fetal bonding and also allows colostrum to supplement the baby’s developing immunity.11,28,33-35,43-45 ASMs are divided into three categories: “safe,” “moderately safe,” and “may be hazardous.” ASM levels in breast milk are significantly lower than concentrations or exposure in utero, and are more variable, with approximately half of all ASMs concentrations lower than detectable limits.11,28,33-35,43-45 As alluded to above, monitoring for baby irritability and sedation would be advised if benzodiazepines or barbiturates are used.33-35,43,52-58
Numerous articles have cited the benefits of breastfeeding and indicate that no significant overall adverse effects of ASM exposure via breast milk appear to have been observed. Furthermore, in the Meador et al.53 study, breastfed children exhibited higher IQ and enhanced verbal abilities.52-58 The exact mechanisms for the findings, while encouraging, however, are not yet known.
TERATOGENESIS AND WOMEN WITH EPILEPSY
The literature indicates that the risk of birth defects in babies from mothers not taking ASMs is approximately 2–4 %. In contrast, babies born to mothers who take ASMs have a risk of approximately 4–8%, with increased risks associated with polypharmacy and higher medication doses.59-61 Major abnormalities include facial clefts, congenital heart disease, and neural tube defects, which can be diagnosed with approximately 95% accuracy by Week 16 of gestation with ultrasound and alpha-fetoprotein screening.59-61 Minor abnormalities, such as ocular hypertelorism and nail hypoplasia, among others, occur at twice the baseline rate, and the incidence is estimated to be approximately 5–20 % in babies born to mothers taking ASMs, according to the literature.59-61
The ACOG has recommended folic acid to mitigate the incidence of neural tube defects (such as encephalocele, meningoencephalocele, and other major malformations). Based on the literature, women of childbearing age without a diagnosis of epilepsy are recommended to take a minimum of 400 mcg of folate daily when not on ASMs. For those taking ASMs, the recommended daily dose is 1 mg of folic acid. Furthermore, when actively planning a pregnancy while on ASMs, the folate dose should be increased to 4 mg daily approximately 3 months prior to pregnancy. European guidelines recommend up to 5 mg of folate per day.12-14,37-43
Folate metabolism may be affected by ASMs, and vice versa. ASMs such as phenytoin can lower folate concentrations, and in cases of malabsorption, approximately 1% of patients may be at risk of megaloblastic anemia.48 Folate may lower phenytoin concentration, at least transiently, so breakthrough seizures may occur in patients who suddenly start taking folate, which is added to their regimen after well-intentioned prenatal counseling.37-43
Folate metabolism is involved in the cytochrome p450 enzyme-mediated conversion or hydroxylation of phenytoin. Adding folate seems to increase phenytoin metabolites, leading to reduced serum levels. Similar effects may occur with carbamazepine.37-43 Gabapentin, lamotrigine, and other medications, however, have minimal effects on folate metabolism.32-35,37-43
See Table 1 for an overview of important drug characteristics in women with epilepsy.
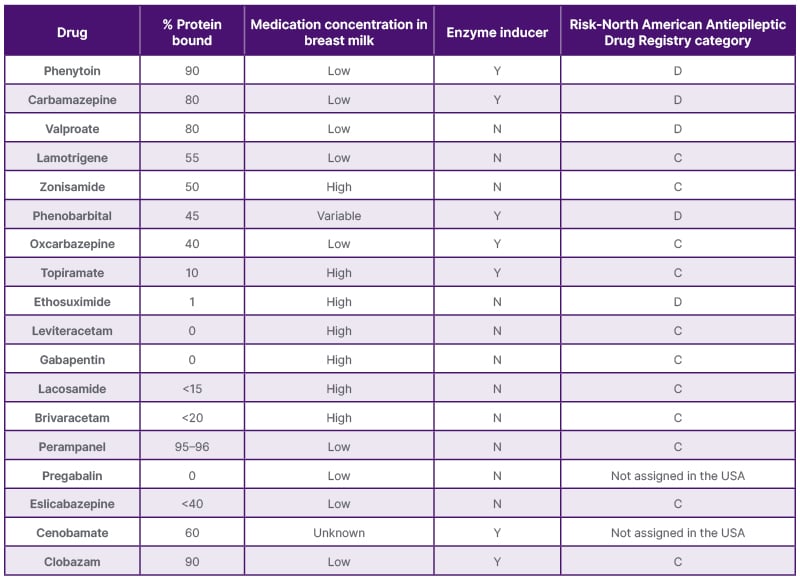
Table 1: Important drug characteristics in women with epilepsy.
BONE HEALTH AND MENOPAUSE
Osteoporosis
Osteoporosis is defined when bone mineral density is less than 2.5 standard deviations below compared to what would be expected.62-64 Osteopenia occurs when bone mineral density is between 1.0 and 2.5 standard deviations below the expected norm.62-64 Decreased bone mass or bones with an abnormal matrix or architecture are more prone to fracture. ASMs that contribute to bone turnover or relative osteoclastic activity (osteoclasts break down bone, while osteoblasts build bone and may become less active with aging and other factors) pose a risk of reducing bone mass or density, particularly in women who take these medications chronically.62-64
Women have less peak bone mass by age 20 compared to men. Approximately 40% of White women will have a fracture related to decreased bone mass as they age. Risk factors include low body weight, lack of weight-bearing exercise, and the hormonal changes of perimenopause. Over their lifespan, women have a threefold higher risk of osteoporotic fractures compared to men, and may lose about 4% of their bone mass each year.62-64 It is estimated that up to 10% of the skeleton may be undergoing remodeling at any given time, with osteoblast activity decreasing with age.62-64 Therefore, due to these noted factors, women generally have lower bone mass and a higher risk of developing osteoporosis and osteopenia. This risk is potentially greater in women with epilepsy, as ASMs commonly increase vitamin D metabolism. As a result, there may be decreased calcium absorption and reduced cellular responsiveness, which can lead to a relative increase in parathyroid hormone, promoting osteoclastic activation and bone resorption.62-64 Therefore, the biochemical and metabolic changes in women with epilepsy, due to ASMs and the chronic nature of treatment, are associated with risks such as hypocalcemia, hypophosphatemia, low vitamin D levels, elevated alkaline phosphatase, and increased parathyroid hormone levels, as noted above.62-64 Screening for osteoporosis in high-risk individuals is recommended, and treatment with weight-bearing exercise, calcium 1000–1500 mg/day, and vitamin D 400–800 IU/day would be recommended, particularly for women taking ASMs that induce liver metabolism. These medications may lead to the above metabolic cascade, posing the risk of bone loss.62-64
GENETICS AND INHERITANCE
Risks of Seizures in Children Born to People with Epilepsy
Patients often inquire about the risk of epilepsy in their children, yet a large amount is still unknown about this expanding field. The inheritance of epilepsy is incompletely understood and complex, with the greatest risk observed in individuals with known genetic syndromes.65-70
The literature indicates that such risks are greater compared with the general population, and could be up to twofold when the parent with epilepsy is the mother. These risks are most pronounced in idiopathic epilepsies, where a genetic mechanism is present or suspected, rather than in symptomatic epilepsies.65-70
In primary generalized epilepsy syndromes, where thalamocortical circuits may be impacted most significantly, there is an estimated 15–40% risk of relatives having childhood absence epilepsy.65-70 Juvenile myoclonic epilepsy may be associated with multiple chromosomal abnormalities.65-70 Familial temporal lobe epilepsy may be, in some cases, autosomal dominantly inherited with 60% penetrance, and in autosomal dominant nocturnal frontal lobe epilepsy, there may be up to 75% penetrance, from a review of the literature.65-70
SEXUAL DYSFUNCTION AND SEIZURES
Literature indicates that overall, there is decreased sexual desire, increased dysmenorrhea with anovulatory cycles, an impact on GnRH, association with seizures, early menopause, and the association of polycystic ovary syndrome.71,72 Other associated risks involving seizure disorders include mood disorders, increased anxiety, and impaired social functioning with negative effects on self-esteem.71,72 In males, lower birth rate and decreased libido and testosterone impaired spermatozoa; however, this could be due to confounding medication issues.73
MENOPAUSE
Literature suggests that seizures may affect GnRH release in the hypothalamus, which may account for women with epilepsy having lower fertility, a higher frequency of anovulatory cycles, and earlier menopause.74,75 Catamenial seizures tend to increase in the perimenopausal period, but reduce post menopause and, in general, since estrogens (or an increased E/P ratio) might induce seizures, non-estrogen-based supplementation may be recommended.74,75
SPECIAL CONSIDERATIONS IN MALES WITH EPILEPSY
Males with epilepsy are less likely to father a child compared to controls, and this may be due to decreased potency and libido, decreased testosterone, or decreased sperm count and motility due to reduced effects of testosterone, or possibly direct effects of medications such as valproate or carbamazepine.73
FUTURE DIRECTIONS AND CURRENT GAPS IN UNDERSTANDING
Although there are currently multiple medications available, and there may be imminent newer medications, technology such as responsive neurostimulation is an available option to treat intractable epilepsy. Responsive neurostimulation was approved by the FDA in 2013, and real-world experience in post-marketing retrospective observational studies has indicated that there are women who have become pregnant after implantation.76,77 The small and limited studies available so far indicate that there does not appear to be any increased risk of malformation or significant impact on the fetus.77 Whether such technology might, in some cases, lead to significant medication reduction, and therefore reduce the risks associated with polypharmacy in pregnancy, such as fetal malformations and challenges with seizure control, remains to be seen, as this was not studied in the pivotal trials that were conducted for authoritative approval. Similarly, future precision medicine might be able to provide specific, effective, and targeted treatment options with the potential to incrementally enhance the diagnosis path, treatment trajectories, and outcomes for patients.78 Exactly which genetic analyses, panels, or tests are indicated for comprehensive risk assessment and therapeutic decision-making in a given patient remains uncertain. In the United States, such testing is often not reimbursed by healthcare insurance providers, particularly since, even when genetic profiling is performed, it may not yield definitive therapeutic options beyond initiating conventional ASMs. The author speculates that testing is currently highly variable among epilepsy centers.
There is little research on new-onset seizures during pregnancy, although limited literature exists, and popular beliefs are noted on the internet.79-82 There may be conflicting evidence regarding the incidence of seizures during labor and parturition. It has been believed or speculated that parturition itself is generally associated with rare or well-controlled seizures, although seizures can occur during labor and delivery by an unknown mechanism. Conditions such as eclampsia or vasoconstriction syndromes, which have a proclivity to occur during this time, are also associated with significant comorbidities and mortality.79-83 Further studies could clarify risks and best practices, potentially having practical implications on, for example, whether or not to proceed with an evolving labor versus planning, or converting to, a cesarean section when risks surrounding labor and parturition and seizures are better understood.
Studies cited above note that seizure risks during pregnancy and the postpartum period may increase or decrease due to significant fluctuations in drug levels. These fluctuations can be influenced by changes in volume of distribution, metabolism, and pharmacokinetics, with both maternal and fetal systems contributing individually and collectively to these factors.32-35 The binding of ASMs to albumin and proteins impacts ASM blood levels. Therefore, measuring free levels may be useful in managing select patients on ASMs that are highly protein-bound. Further literature may help clarify when such measurements are most beneficial.32-35 Furthermore, ASMs may impact levels of sex hormone-binding globulin, which could influence libido and fertility, and this could be a subject for significant further study.32-35,82,83,84
A small amount of literature exists that documents patient-centered fears that anti-seizure medications themselves may confer the risk of seizures.85 The authors of this study speculated that, while genetic factors likely play a significant role in the development of epilepsy, prenatal ASMs exposure might exhibit a causative role, and further research would be useful in this regard.85
Significant confounding sleep disorders that occur concurrently may impact the occurrence of seizures in relation to the reproductive lifespan, and this connection is becoming increasingly accepted.86-89 Nonrestorative sleep increases risks of seizures, although the exact biological mechanism is unknown.87-89 Processes that increase nonrestorative sleep, or fragment sleep, are caused by various issues and diagnoses.86-89 Such issues and diagnoses may include weight gain, which can cause or contribute to obstructive sleep apnea and seizures, as well as restless leg syndrome, periodic limb movements in sleep, and nocturnal seizures, which might also further fragment sleep and increase seizures.86-89
The optimal timing for assessing a pregnant patient for obstructive sleep apnea and determining the appropriate treatment, such as continuous positive airway pressure titration, which may result in a fixed prescription based on airway resistance, weight, and the size of the gravid uterus, has not yet been clearly defined in clinical practice. Additionally, the use of automatic, adjustable continuous positive airway pressure devices, which adjust depending on detected flow limitation, may also be considered. However, due to the changes in airway resistance caused by increased weight and the growing uterus, as well as the effects on respiratory mechanics and airway collapse during pregnancy, further studies are needed to optimize these approaches.87-89
There may be non-pharmacological options of significant utility for the management of patients and their babies when the patient has a seizure disorder. Newborn safety is a significantly understudied issue.81 The author suggests that it may be beneficial to advise changing the height and location of the baby’s feeding and care area, keeping it low to the ground. This precaution would reduce the risk of injury to the baby in the event of a maternal seizure during activities such as diaper changing, dressing, or feeding, as it would help prevent the baby from falling and sustaining a traumatic injury. The author also advocates for general awareness and assessment of safety in the home to be considered from the vantage point of how to best keep the mother and baby safe in case the patient experiences altered awareness. Exactly how wearable, or other portable technologies in the home, might be useful is yet unknown; however, the author shares an example of a patient in which a case of parasomnia, rather than a seizure, was identified and detected using a home security camera system. This allowed for further evaluation and helped clarify the situation. Additionally, the author has advised involving the patient’s partner to help facilitate continuous sleep to mitigate the risk of seizures. The author has also recommended maternal milk harvesting and storage as a strategy, allowing the partner to assist in feeding, which could help women with epilepsy achieve uninterrupted sleep during the night. This approach may reduce overall seizure occurrences and support breastfeeding, ensuring the infant can still consume breast milk during the night if needed. However, comprehensive studies on these practices remain relatively anecdotal.
Although there is scarce literature, the author advises that, in appropriate cases, excluding psychogenic nonepileptic seizure disorders may be useful and should potentially be pursued in an epilepsy monitoring unit stay to eliminate or minimize the risk of ASM-mediated teratogenicity. Evaluation should be undertaken in a timely manner, before conception, to prevent unnecessary ASM exposure to a developing fetus. However, there is limited literature and guidance currently available on this important topic, and the incidence of ASM exposure in psychogenic nonepileptic seizure patients and their fetuses is unknown, even among those practicing in epilepsy centers.90
Although there is an extensive understanding of the hormonal influences of menstruation and CE, the full extent of the evaluation and management of increased seizures with in vitro fertilization is not known, although the literature is evolving and could be a practical course of study.91
Generally, the current literature advocates for mitigating GTCSs, as they may carry the highest comorbidities and potential effects on uteroplacental insufficiency and the risk of abruption by the third trimester of pregnancy. However, it remains unknown how or to what extent other seizure types might impact maternal-fetal insufficiency and maternal-fetal physiology, requiring further management and investigation.32-35
There are no guidelines regarding whether medication reduction or down-titration strategies to mitigate exposure during the first trimester might reasonably be undertaken in certain, well-defined circumstances. From the author’s experience, some patients often insist on or request such maneuvers, even when seizures have occurred very remotely, sometimes decades ago. However, the current practice rationale does not specify what to do in these circumstances. The general tendency seems to be to continue ASMs anyway, aiming to achieve the best seizure control possible despite the potential risk of ASM exposure to the fetus.32-35 Currently, although there is a consensus in aiming not to have risks of generalized seizures by the third trimester, to prevent placental abruption and/or placental fetal insufficiency, the issue of whether or not to taper medication in those with what might be considered an “outgrown” seizure disorder as part of preconception counselling is not fully clarified in the literature.32-35
Finally, there are no comprehensive consensus guidelines on how to optimally manage patients with intractable seizures having uncontrolled seizures during pregnancy. This applies to patients who are not seizure-free and require medication changes or additional adjunctive medications, or those who have already transitioned to second-line therapy but continue to experience seizures due to conditions like idiopathic generalized epilepsy. While there are evolving articles on managing these “second-line treatment” options or adjunctive ASM management for seizure control, newer non-hepatic enzyme inducers such as levetiracetam and lamotrigine show promise. Among non-valproic acid add-on regimens, the combination of levetiracetam and lamotrigine may demonstrate superior effectiveness compared with other ASM combinations in certain cohorts of women.32-35,92 Significantly more studies and treatment options are needed for this specific cohort of patients.







