Authors: *William Busse,1 Geoffrey Chupp2
1. Division of Allergy, Pulmonary and Critical Care Medicine, University of Wisconsin School of Medicine and Public Health, Madison, USA
2. Division of Pulmonary, Critical Care and Sleep Medicine, Yale University School of Medicine, New Haven, Connecticut, USA
Disclosure: Busse has received honoraria from AstraZeneca, GlaxoSmithKline (GSK), Regeneron Pharmaceuticals, and Sanofi-Genzyme for speaking and consultancy services; and has held grants from National Institutes of Health (NIH)-National Institute of Allergy and Infectious Diseases (NIAID) and NIH-National Heart, Lung, and Blood Institute (NHLBI). Chupp has received honoraria from GSK, AstraZeneca, Genentech, Novartis, Sanofi-Genzyme, Regeneron Pharmaceuticals, and Teva Pharmaceuticals.
Acknowledgements: Medical writing assistance provided by Caroline E. Cross, Reading, UK.
Disclaimer: The opinions expressed in this article belong solely to the named speakers. For mepolizumab prescribing information, please navigate to the appendix at the end of the article.
Support: The symposium and publication of this article was supported by GSK Respiratory
Keywords: Asthma, biologics, exacerbations, lung function, remission, severe asthma.
Citation: EMJ Respir. 2023;11[Suppl 1]:2-10. DOI/10.33590/emjrespir/10306104. https://doi.org/10.33590/emjrespir/10306104.
Meeting Summary
Asthma affects more than 250 million people worldwide, and is responsible for almost half a million deaths and more than 21 million disease-adjusted life years annually.1 It is a chronic inflammatory condition that can lead to progressive loss of lung function and comorbidities related to short-term and prolonged use of oral corticosteroids (OCS).2,3
Treatments for many chronic inflammatory conditions have evolved in recent years, and novel therapeutics such as biologics are showing real promise in improving patients’ quality of life.4,5 In rheumatoid arthritis (RA), for example, physicians and patients are increasingly looking beyond symptom control to disease remission as a treatment goal.4
Despite asthma’s distinct pathophysiology, there is now evidence that, like RA, early diagnosis and treatment in asthma may slow, and potentially prevent, disease progression, making the prospect of disease remission for asthma a possibility.5,6
During this sponsored symposium, held as part of the American Thoracic Society (ATS) Meeting in Washington, D.C., USA, asthma experts William Busse, Division of Allergy, Pulmonary and Critical Care Medicine, University of Wisconsin School of Medicine and Public Health, Madison, USA; and Geoffrey Chupp, Division of Pulmonary, Critical Care and Sleep Medicine, Yale University School of Medicine, New Haven, Connecticut, USA, discussed clinical remission and its potential positioning in asthma, and as a treatment goal. They outlined their visions and ambitions for remission as a treatment goal for patients with asthma, and described the pathway to realising this ambition.Introduction
For many people living with asthma, medications such as inhaled corticosteroids (ICS) and short- or long-acting β agonists are sufficient to control symptoms, which include airway constriction, breathing difficulties, and limitations to a normal lifestyle.3,7 However, asthma is a chronic inflammatory condition, prone to exacerbations, which negatively influence patients’ lung function, symptoms, and quality of life. Among patients with asthma, 3–10% of patients experience severe asthma that, despite treatment, often remains uncontrolled, with frequent exacerbations.5
The Global Initiative for Asthma (GINA) regularly updates asthma treatment guidelines and recommends the use of ICS to reduce the risk of exacerbations.3,7 However, many patients, who are not adequately controlled with ICS plus an additional controller frequently receive maintenance and/or recurring bursts of OCS. Such exposure to corticosteroids is associated with long-term side effects and comorbidities, such as osteoporosis and cardiovascular disease, that further affect quality of life.2,8
The treatment landscape for severe asthma is changing with the advent of new and emerging therapies that hold promise for improving treatment goals and patient outcomes.9 In this sponsored symposium, asthma specialists Busse and Chupp outlined how treatments for other inflammatory conditions have evolved to make remission a realistic treatment goal for patients, and discussed what needs to happen now to turn the aspiration for clinical remission for asthma patients into a reality.
What Is Clinical Remission?
Geoffrey Chupp
Clinical remission is not a new concept in disease management. It is a commonly used term in cancer, diabetes, and inflammatory conditions such as RA.4,10,11 Definitions vary depending on the symptoms and underlying pathophysiology of the disease in question, but, in general, remission occurs when signs and symptoms have decreased either to a significant degree or completely, and the quality of life has returned to normal.4,10,11
Chupp opened the session by asking the audience whether they believed in the concept of clinical remission for severe asthma. A majority (62%) said they did, whilst 36% were still unsure.
“We want to introduce remission as a therapeutic concept and goal in asthma management. We want to define some of the characteristics, provide a rationale for remission, and present a case for its value for patients. Ideally, we want to be effective in supporting patients to lead to a more normal lifestyle, and making asthma remission an important component of the patient treatment paradigm,” Chupp said.
For RA, early diagnosis in specialist clinics has become an important step in improving treatment success. Chupp went on to explain: “In the 1990s, treatments for RA changed dramatically with the introduction of biologics.”12 Moreover, the concept of treatment changed when patients were seen early in the course of RA and promptly treated with biologics, which were found to modify the course of disease and quell inflammatory changes.13,14
Chupp went on to explain how rheumatologists put forward the hypothesis that they were driving the disease into remission: “The characteristics of having the disease [RA] were there, but the disease endpoints of systemic inflammation and tissue destruction were quelled.”
Although asthma and RA have distinct underlying disease mechanisms, they are both chronic inflammatory conditions that are considered incurable. “Both diseases can progress if left unchecked,” Chupp explained, adding: “In RA, damage occurs in the joints and results in visible disability, whereas in asthma, exacerbations can lead to progressive and irreversible loss of lung function and a vicious cycle of disease, which is underestimated because it is not readily visible.”14-16
The advent of new targeted treatments is making clinical remission a realistic goal for RA, and Chupp believes that, as appropriate endpoints for disease control and patient outcomes are identified and defined, it is now possible to explore the feasibility of achieving clinical remission in severe asthma, too.14,17,18
The Unmet Medical Need: Why Is Clinical Remission Important?
Geoffrey Chupp
Current guidelines for asthma treatment recommend that physicians and patients aim for adequate symptom control and reducing the risk of exacerbations (Figure 1).19 In addition, within the guidelines, maintenance systemic corticosteroids are recommended as an ‘other controller option’ for people whose symptoms remain uncontrolled despite first-line medication, including short-acting β agonists or long-acting β agonists and ICS. Chupp believes that physicians and patients should raise their ambitions for a greater comprehensive level of asthma control.
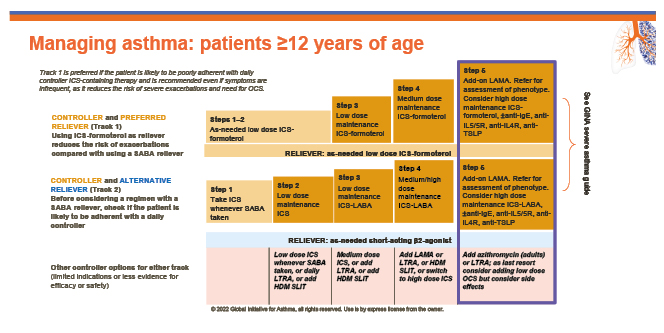
Figure 1: The Global Initiative for Asthma’s five-step treatment guidelines.19
GINA: Global Initiative for Asthma; HDM SLIT: house dust mite sublingual immunotherapy; ICS: inhaled corticosteroid; LABA: long-acting β agonists; LAMA: long-acting muscarinic antagonist; LTRA: leukotriene receptor antagonist; OCS: oral corticosteroid; R: receptor; SABA: short-acting β agonists; TLSP: thymic stromal lymphopoietin.
“We need to aim for optimal control as an endpoint, not just adequate control,” asserted Chupp. “We have lots of therapeutic options, which is wonderful, but we need to move ahead and think about a future where we identify patients and the factors required to achieve optimal control.”
Both Chupp and Busse are convinced that with the availability of effective biologics for asthma treatment, optimal control is an achievable goal. “As a community, we need to set that as our goal, otherwise it will be hard for us to get there,” Chupp emphasised.
Chupp also pointed out that ongoing exposure to systemic corticosteroids places a huge burden on people with asthma. “Cumulative exposure to corticosteroids increases risk factors for diabetes, osteoporosis, progressive loss of lung function, and cardiovascular events.”20 A long-term observational study of historical matched patient data (n=24,117) demonstrated that adverse outcomes began at cumulative exposures exceeding 0.5 g of corticosteroids.20 Chupp explained that this is not an unusual level of exposure, which means patients who experience just one exacerbation per year have an increased risk of comorbidity. “Exacerbations can accelerate and escalate, and result in loss of lung function and activation of Type 2 inflammation,” Chupp said, which makes the condition more difficult to control.21
Clinical data also highlight that the impact on lung function decline is strongest in 18–39-year-old patients, suggesting earlier intervention in the asthma patient journey would be beneficial.22 “By keeping the disease ‘quiet’ for longer periods of time, we can improve disease activity, and this could potentially be disease-modifying,” Chupp enthused.
Eosinophilic inflammation correlates with loss of lung function and is one of the best available biomarkers for predicting disease progression and risk of exacerbation.21 Higher levels of sputum eosinophils are associated with a greater rate of lung function decline. “Indeed, an association has been demonstrated between ‘fast’ decliners or ‘slow’ decliners, and the respective levels of eosinophil cationic protein in sputum,” Chupp explained.23
Patients’ dependence on OCS also remains a key challenge in severe asthma management, as approximately 50% of patients with severe asthma are managed with maintenance or recurrent OCS, exposing them to long-term risks of OCS adverse events, despite calls for OCS-sparing treatment strategies.2,20,24,25
This further demonstrates the huge unmet clinical need, Chupp said. “We are fortunate to be able to start conversations and address these unmet needs, thanks to the biologics we now have available to use.”25
Defining More Disease-Specific and Relevant Endpoints for Patients with Severe Asthma
William Busse
Busse explained that, to set a goal of disease remission for asthma, endpoints that are disease-specific and relevant must be defined and measured. Busse asked the audience which endpoint they would consider most important in a definition of asthma remission. A large proportion of the audience (40%) considered ‘exacerbation-free’ to be the most important endpoint to consider, with 30% saying ‘symptom control’. A smaller proportion of the audience (20%) thought ‘OCS-free’ was the most important endpoint, and 10% said ‘maintenance or improvement of lung function’.
Busse went on to outline how they had convened as part of a panel of specialists 3 years ago to consider what the characteristics of remission should be for asthma. The expert group used a modified Delphi survey approach to identify core components of asthma remission as a treatment goal to develop a consensus framework, and included their personal opinions and experiences, and evidence from the published literature. The generalised framework provides an outline for a definition for clinical remission and complete remission of asthma, on and off treatment (Figure 2).18
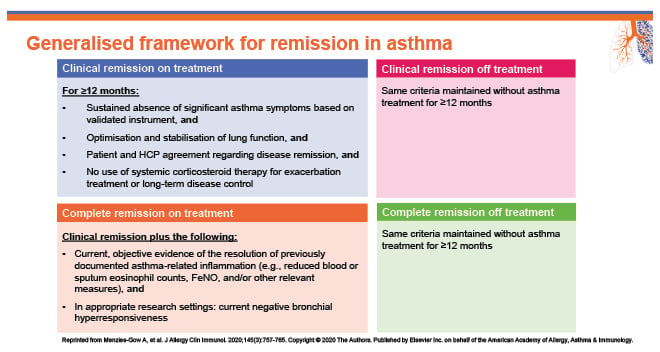
Figure 2: A generalised framework for remission in asthma.18
FeNO: fractional exhaled nitric oxide; HCP: healthcare professional.
The expert panel believe that remission should include a holistic view of the state of disease, and include more than one endpoint in its definition.18
“This is a wish at present. We are not quite there yet,” emphasised Busse, “but we should aim for remission in some cases and eventually cure.” They added: “Exacerbations set the stage for disease progression, so one of the future treatment goals should be ‘exacerbation-free’.” Busse went on to outline the four endpoints that could be used to assess the potential to achieve remission with current therapeutics: exacerbation-free, OCS-free, symptom control, and lung function optimisation/stabilisation.18
Treatment recommendations have evolved. Twenty years ago, guidelines recommended rapid intervention with OCS for asthma exacerbations.18 “We wanted to make people safe, and OCS helped achieve that,” Busse said. “However, now, data suggest that multiple courses of systemic corticosteroids in a patient’s lifetime pushes them into areas where adverse sequalae of these treatments can be quite significant, affecting bone structure, metabolic, and cardiovascular events.”2,8 Both speakers believe that physicians should now try to avoid OCS use as much as possible, and OCS-free should be an important outcome to consider as part of defining remission.
“In addition, it is now clear that repeated exacerbations are more likely to lead to progressive loss of lung function,” Busse continued. “Many patients with severe asthma have a forced expiratory volume below 60%. By this stage, they have remodelling in the lungs, and recovering normal level lung function is very difficult to achieve.”
Busse went on to explain the importance of preventing disease progression: “To help prevent progression, one of our treatment goals should be stabilising lung function.” However, as Busse elaborated, the criteria for remission are still being evaluated, and further work is needed to understand whether they will translate into improved outcomes for patients. “We have done 1 year of analysis, but the longer we maintain remission, the better our insights as to whether the benefits in managing the disease will be long-term.”18
Research is also underway to identify predictors of asthma remission versus persistence based on baseline patient characteristics. As part of the ADONIS study, 200 adults with a recent diagnosis of asthma were followed prospectively for 5 years.26 Patients were considered to be in remission if their symptoms were absent and they had not used asthma medication for a year or more during the 5-year follow-up period. The researchers found that 16% of patients experienced asthma ‘remission’. They identified predictors of remission to include a younger age, better asthma control, lower ICS dose, less severe airway hyper-responsiveness, less nasal polyps, and lower levels of blood neutrophils.26 “Although this study did not look at patients with severe asthma, it does provide data on who is most likely to have spontaneous remission. Where there is less injury and less disease activity, you are more likely to be able to get the disease under control,” Busse added. “We want to be able to identify patients who are at a higher risk of experiencing exacerbations and intervene early with standard therapies and, in some cases, biologics.”
To support this approach, the ORACLE prognostic risk score may help predict prognosis, and could potentially inform guidelines for remission.27 Risk factors are defined by current GINA guidelines, and an Asthma Control questionnaire used to identify patients at risk of exacerbations. This tool can also identify patients in the ‘moderate’ disease group, who are at high risk of exacerbations based on their biological phenotype, including high eosinophil count.28
Where Do We Go from Here?
Geoffrey Chupp
“We have an opportunity to aim for remission, rather than just control, and we should embrace it,” Chupp reiterated. They believe that updating treatment guidelines to include the goal of achieving remission is critical, despite the associated challenges.3 “We must engage patients as well as providers. We need to recognise what our goals are so we can disseminate that information, and so that patients do not have to experience the burden of symptoms on a regular basis.”
In addition, Chupp said we need more research on the concept of remission and how we get there. “We have some amazing therapeutics coming through. As we understand more about the biology of asthma, we can reach our ultimate goal of achieving a cure in some patients.”
“Firstly, we need to go for optimal symptom control. OCS freedom should be prioritised.17,29,30 Secondly, early evaluation and intervention is key to preventing irreversible lung damage.29 Thirdly, we need disease modifying strategies that may offer improved lung function for patients.29,30 If we reduce exacerbations, we will help slow disease progression, and in a way that is a disease modifier,” Chupp said.
Shared decision-making for asthma management is also important, and has been shown to improve adherence, outcomes, and patient satisfaction with care.31,32 “Our remission framework highlights the need for patient and healthcare professionals to understand, appreciate, and agree with the concept and benefit of aiming for remission, but additional work is required to determine how that agreement is best evaluated.”18 Chupp further explained: “We need to encourage more physicians to have conversations with their patients about what remission would entail in relation to asthma.”
Both speakers believe that remission for severe asthma should be an aspirational, intentional goal to optimise/stabilise lung function, reduce adverse effects associated with OCS, and reduce exacerbations. For some patients with severe asthma, remission may be an achievable goal that can improve their disease and reduce treatment burden, while minimising the effects of disease progression.33 Researchers and key organisations are now starting to apply the concept of remission and are actively working to define clinical remission criteria. They are also using clinical trials to identify predictors of remission.33 Further validation of predictors of remission and earlier use of biologics are critical to achieve this aim.34,35
Biologics: Pushing Forward the Frontier to Remission and Cure
Geoffrey Chupp
The symposium concluded with a question-and-answer session where participants were keen to know more about the role of biologics in reducing asthma disease burden. Chupp explained how some clinical trials have shown that 20–25% of patients with severe asthma achieve at least some of the criteria for clinical remission. However, the endpoints utilised are not yet standardised.33 Chupp continued: “Biologics as monotherapy is intriguing, but we need to generate more evidence. How we design suitable clinical trials is critical.”36 Chupp believes more industry-supported trials are needed where patients with moderate asthma are included to see whether they become inhaler- and steroid-free, thereby achieving remission. “Within trials, we need to treat-to-failure rather than treat-to-target, to measure remission as the endpoint,” they said and, if remission is possible, we can then look for a step down in medications, “but we need more evidence.”
“In the next 5 years, we should see changes in our treatment approach. We need to recognise that asthma is a complex disease. We need to phenotype patients as soon as they come in, define risk markers, and treat-to-control very quickly, to slow disease progression and increase the chance of achieving remission,” Chupp said.
Speaking after the symposium, Chupp described data presented in a poster at the meeting. They said: “To test the hypothesis that a proportion of patients with severe asthma can achieve multi-component remission, a team led by [Guy] Brusselle at Ghent University, Belgium, is carrying out a real-world analysis of data from a clinical trial called REALITI-A.”37 The REALITI-A trial aimed to assess the effectiveness of mepolizumab, approved for the treatment of severe eosinophilic asthma, in reducing the need for OCS use in patients with severe asthma.37 “What we see in this post hoc analysis is that after 1–2 years of treatment with mepolizumab, approximately one-third of patients met the three-component definition of clinical remission, being maintenance OCS- and exacerbation-free with controlled symptoms (29% at Week 52; baseline 2%; n=214; and 33% at Week 104; baseline 1%; n=184, respectively).38 When we include lung function as a fourth criterion for clinical remission, we find that the number of people achieving the four-component definition of remission increased to 35% at Week 52; baseline 4%; n=74; and 37% at Week 104; baseline 2%; n=52, respectively.”38
“This data is really encouraging and further suggests that clinical remission is a realistic goal for at least a subset of patients with severe asthma, following treatment with biologics. The earlier we can intervene in the course of disease, the more successful we are likely to be in slowing progression, and driving the disease into remission,” Chupp concluded.
Nucala (mepolizumab) Prescribing information Great Britain (GB)
(Please consult the full Summary of Product Characteristics for Great Britain (SmPC GB) before prescribing)
Presentation: Nucala (mepolizumab) solution for injection in pre-filled pen. Each 1 ml pre-filled pen contains 100 mg mepolizumab. Nucala solution for injection in pre-filled syringe. Available as 100 mg solution for injection in a pre-filled syringe. Each 1 ml pre-filled syringe contains 100 mg mepolizumab. Available as 40 mg solution for injection in pre-filled syringe. Each 0.4 mL pre-filled syringe contains 40 mg of mepolizumab. Nucala powder for solution for injection. Each vial contains 100 mg mepolizumab. After reconstitution, each 1 ml of solution contains 100 mg mepolizumab. Indication: Severe eosinophilic asthma: Indicated as an add-on treatment for severe refractory eosinophilic asthma in adults, adolescents and children aged 6 years and older. Chronic rhinosinusitis with nasal polyps (CRSwNP): indicated as an add-on therapy with intranasal corticosteroids for the treatment of adult patients with severe CRSwNP for whom therapy with systemic corticosteroids and/or surgery do not provide adequate disease control. Eosinophilic granulomatosis with polyangiitis (EGPA): indicated as an add-on treatment for patients aged 6 years and older with relapsing-remitting or refractory eosinophilic granulomatosis with polyangiitis. Hypereosinophilic syndrome (HES): indicated as an add-on treatment for adult patients with inadequately controlled hypereosinophilic syndrome without an identifiable non-haematologic secondary cause
Posology and method of administration: Severe eosinophilic asthma: Nucala should be prescribed by physicians experienced in the diagnosis and treatment of severe refractory eosinophilic asthma, CRSwNP, EGPA or HES. For severe refractory eosinophilic asthma: Adults and adolescents aged 12 years and over: Recommended dose is 100 mg administered subcutaneously once every 4 weeks. Children aged 6 to 11 years old: Recommended dose is 40 mg administered subcutaneously once every 4 weeks. For CRSwNP the recommended dose in adults is: 100 mg of mepolizumab administered subcutaneously once every 4 weeks. For EGPA the recommended dose for adults and adolescents aged 12 years and older is 300 mg administered subcutaneously once every 4 weeks. Children aged between 6 and 11 years old: Children weighing ≥ 40 kg recommended dose is 200 mg administered subcutaneously once every 4 weeks. Children weighing < 40 kg recommended dose is 100 mg administered subcutaneously once every 4 weeks. HES recommended dose in adults is 300 mg administered subcutaneously once every 4 weeks. Treatment is intended long-term and need for continued therapy should be considered at least annually. Administration is by subcutaneous injection only. Powder for solution for injection: Should be administered by a healthcare professional. Requires reconstitution. Each vial should be used for a single patient, and any remainder of the vial should be discarded. Solution for injection in a pre-filled pen and pre-filled syringe (100 mg): May be self-administered by the patient or administered by a caregiver if their healthcare professional determines it is appropriate, and patient/caregiver are trained in injection techniques. Pre-filled syringe (40 mg) – must be administered by a healthcare professional or a caregiver. Please see package leaflet for instructions on administration. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Warnings and precautions: Name and batch number of the administered product should be recorded in patient file. Not to be used to treat acute asthma exacerbations. Asthma-related adverse symptoms or exacerbations may occur during treatment. Abrupt discontinuation of corticosteroids after initiation of therapy is not recommended. Hypersensitivity and administration-related reactions have occurred following administration, generally within hours of administration, but in some instances, they may have a delayed onset (i.e. typically within several days). These reactions may occur for the first time after a long duration of treatment. Pre-existing helminth infections should be treated before commencing Nucala. If patients become infected and do not respond to anti-helminth treatment, temporary discontinuation of Nucala should be considered. Nucala has not been studied in patients with organ threatening or life-threatening manifestations of EGPA. Nucala has not been studied in patients with life-threatening manifestations of HES Special populations: No dose adjustment is required in elderly patients, patients with hepatic impaired or patients with renal impairment with a CrCl 50-80ml/min. Interactions with other medicinal products: No interaction studies have been performed. Potential for interactions is considered low. Fertility, pregnancy and breast-feeding: Potential for harm to a human foetus is unknown. Preferable to avoid use during pregnancy. Administration should only be considered if the expected benefit to mother is greater than risk to foetus. No data on excretion of Nucala in human milk or on human fertility. Side effects: Very Common (≥1/10): Headache. Common (≥1/100 to <1/10): Lower respiratory tract infection, urinary tract infection, pharyngitis, hypersensitivity reactions (systemic allergic), nasal congestion, abdominal pain upper, eczema, back pain, administration related reactions (systemic non-allergic; most commonly including rash, flushing, myalgia), local injection site reactions, pyrexia. Rare (≥1/10,000 to <1/1,000): anaphylaxis. Please consult SmPC for further information on adverse reactions. Legal category: POM. Presentation and Basic NHS cost: Nucala 1 vial, 1 pre-filled pen, or 1 pre-filled syringe 100 mg – £840.00, or 1 pre-filled syringe 40 mg – £336.00. Marketing authorisation (MA) numbers GB- [vial: PLGB 19494/0285; pre-filled pen: PLGB 19494/0290; prefilled-syringe 100 mg: PLGB 19494/0291; pre-filled syringe 40 mg: PLGB 19494/0303] MA holder: GB- GlaxoSmithKline UK Limited 980 Great West Road Brentford Middlesex TW8 9GS United Kingdom Last date of revision: February 2023, PI-11333. Trademarks are owned by or licensed to the GSK group of companies. © 2018 GSK group of companies or its licensor Nucala.
| Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App Store. Adverse events should also be reported to GlaxoSmithKline on 0800 221 441. |
PM-GBL-MPL-OGM-230001 July 2023







