Abstract
White light cystoscopy (WLC) has long been the standard procedure for visualisation of non-muscle-invasive bladder cancer (NMIBC), both during transurethral resection of bladder tumour (TURBT) and subsequent surveillance visits. The limitations of white light in the operating room are well recognised, and blue-light-guided diagnosis with hexaminolevulinate (HAL) is routinely used as an adjunct to white light rigid cystoscopy to allow for improved detection of malignant tumours. Emerging data for the implementation of blue light flexible cystoscopy (BLFC) in the surveillance setting demonstrate similar clinical benefits to its current use in rigid cystoscopy. In the first Phase III study comparing BLFC with HAL versus WLC for NMIBC surveillance, around 1 in 5 confirmed malignancies were detected only by BLFC (p<0.0001) and the incidence of HAL-related adverse events (AE) was very low. The introduction of BLFC for routine surveillance post-TURBT is supported by consensus among bladder cancer specialists. Patients at high risk of recurrence could benefit from BLFC at the 3 and 6-month cystoscopy, and at 3 to 6-monthly intervals thereafter for the first 2 years post-TURBT. Intermediate-risk patients may also benefit from BLFC at the initial 3-month cystoscopy. Further research is needed to confirm the optimal timing of BLFC in high and intermediate-risk patients, and to clarify the role of BLFC in surveillance of low-risk patients. The ongoing Nordic and USA blue light registries should help to answer these questions.
INTRODUCTION
Surveillance protocols for non-muscle-invasive bladder cancer (NMIBC) involve regular cystoscopic evaluations to detect recurrent disease, which occurs in around 50–70% of cases after successful management of the initial tumour.1 Surveillance cystoscopy is performed primarily in the outpatient setting, with white light cystoscopy (WLC) the long-held standard procedure for tumour visualisation. WLC is a highly sensitive technique for detecting papillary lesions, but suboptimal visualisation of carcinoma in situ (CIS) results in false-negative rates of up to 20%.2,3 Many early recurrences of NMIBC may in fact be missed tumours or result from inadequate resection due to poor visualisation of the initial tumour.4,5 Residual disease rates at sites previously treated by transurethral resection of bladder tumour (TURBT) with WLC range from 27–62%, and up to 10% of cases are missed where NMIBC has progressed to invasive disease.5
Enhanced cystoscopic methods using blue light have demonstrated improved sensitivity for tumours that are missed by white light, and these methods are recommended by international guidelines.6,7 Blue light cystoscopy (BLC) is not a new procedure, and it has been used at the time of TURBT with rigid cystoscopy as an adjunct to the standard white light procedure for the past decade.6 One concern with standard surveillance is that BLC is only used if a patient is referred to the operating room due to detection of a tumour by WLC or due to positive cytology and equivocal lesions on WLC. The implications of missed cancer with WLC in the operative setting is that cancer could also be missed with WLC in the surveillance setting. A clear role has therefore emerged for outpatient blue light flexible cystoscopy (BLFC).8,9 The goal of this article is to discuss the impact of BLFC on the detection and management of bladder lesions and on patient-reported outcomes and acceptability of BLFC surveillance.
BLUE LIGHT CYSTOSCOPY
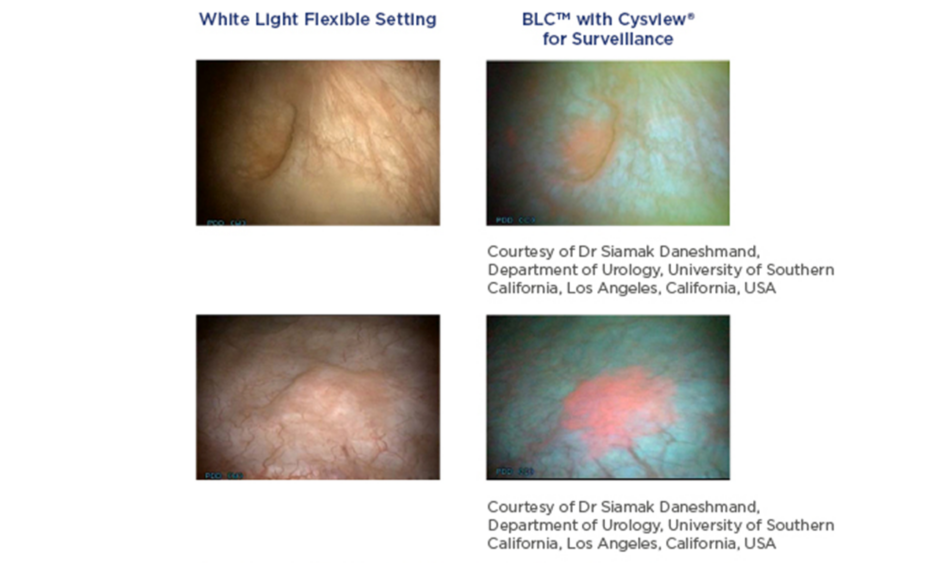
BLC is a photodynamic diagnostic technique that involves intravesical instillation of the heme precursor hexaminolevulinate (HAL) (Hexvix®/Cysview®, Photocure ASA, Oslo, Norway) prior to cystoscopy. This results in the preferential accumulation of protoporphyrin IX and other photoactive porphyrins in neoplastic tissue, which fluoresce red when exposed to blue light (Figure 1).10
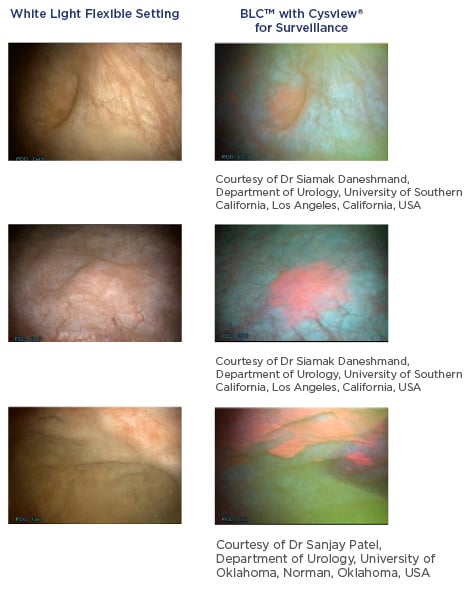
Figure 1: Detection of non-muscle invasive bladder cancer with flexible white light and blue light cystoscopy with hexaminolevulinate.
The majority of BLC experience to date has been in the operative setting, with multiple prospective studies comparing BLC favourably with WLC at the time of TURBT with significantly improved detection of bladder lesions, in particular CIS, Ta, and high-grade tumours.11-15 A 2011 pooled analysis of patient-based data from six studies evaluating BLC with intravesical instillation of HAL reported sensitivities for CIS of 72.2% for WLC and 92.8% for BLC.14 A 2013 meta-analysis by Burger et al.3 found that 26.7% of patients had at least one CIS lesion detected by BLC but not by WLC (p<0.001), with a similarly significant improvement in detection of Ta/T1 tumours with BLC. In both cases, the results were also significant when evaluating the subsets of primary and recurrent tumours.3
Current European Association of Urology (EAU) guidelines recognise the extensive data supporting the use of BLC in the operative setting, stating: “It has been confirmed that fluorescence-guided biopsy and resection are more sensitive than conventional procedures for the detection of malignant tumours, particularly for CIS (level of evidence: 1a). In a systematic review and meta-analysis, photodynamic diagnosis had higher sensitivity than white light endoscopy in the pooled estimates for analyses at both the patient level (92% versus 71%) and biopsy level (93% versus 65%).”7 The guidelines recommend urologists to use methods such as fluorescence cystoscopy or narrow-band imaging to improve tumour visualisation during TURBT, if available. Similarly, the American Urological Association (AUA) and Society of Urologic Oncology (SUO) joint guideline for managing NMIBC recommends that clinicians: “…should offer blue light cystoscopy at the time of TURBT, if available, to increase detection and decrease recurrence. (Moderate Recommendation; Grade B).”15
Detecting more cancers and CIS using BLC can lead to fewer recurrences. Long-term follow-up of 551 patients enrolled in a prospective, randomised study comparing BLC and WLC for Ta/T1 urothelial bladder cancer found that BLC significantly delayed time to recurrence (median 16.5 months versus 9.4 months; p=0.04). BLC also improved the recurrence-free rate, although this was not statistically significant (38.0% versus 31.8%; p=0.14).16
Several studies have since demonstrated similar benefits with recurrence rates at first follow-up cystoscopy and at 2 and 3 years after TURBT were all lower with BLC compared to WLC (Table 1).17-19 A single-centre analysis by Gallagher et al.19 showed that implementing diagnostic BLC as routine practice for all new NMIBC patients significantly reduced 3-year recurrence rates compared with a historical control group of patients who had standard TURBT (39.0% versus 53.3%; odds ratio [OR]: 0.56; 95% confidence interval [CI]: 0.35–090; p=0.02), particularly in high-risk patients (52.1% versus 80.0%; OR: 0.27; 95% CI: 0.10–0.74; p=0.01).19 In a meta-analysis using raw data (n=634), overall recurrence rates up to 12 months favoured BLC (34.5% versus 45.4%; relative risk: 0.761; 95% CI: 0.63–0.92; p=0.006).3 A multicentre registry of patients in the USA with suspected NMIBC undergoing diagnostic BLC is ongoing. Analysis of the first 533 patients (641 BLC procedures) supports improved detection rates of CIS and papillary lesions with BLC versus WLC alone, resulting in a change in management in 14% of cases.20
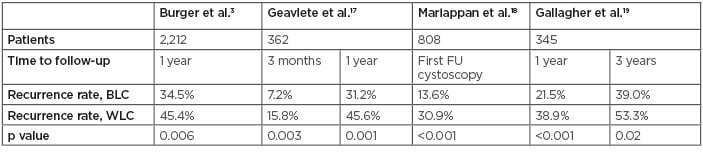
Table 1: Studies comparing recurrence rates using blue light cystoscopy and white light cystoscopy.9
BLC: blue light cystoscopy; FU: follow-up; WLC: white light cystoscopy.
The additional morbidity and mortality of progression is a major concern in patients with high-risk NMIBC, and there is an ongoing effort to determine the impact of blue light procedures in this regard.21,22 A systematic literature review identified four randomised studies and one retrospective study evaluating the impact on progression of BLC versus WLC at the time of TURBT. A total of 1,301 patients were enrolled across the five studies, including 644 patients evaluated by BLC with HAL and 657 patients evaluated by WLC; progression was reported in 44 patients (6.8%) and 70 patients (10.7%), respectively, favouring HAL (OR: 1.64; 95% CI: 1.10–2.45; p=0.01).21 These findings support the design of future randomised trials with progression as a primary endpoint.
For the purposes of future trials, a new definition of NMIBC progression has been proposed by the International Bladder Cancer Group, with the aim of more accurately determining patient prognosis and improving comparisons between treatment options.23 A re-analysis of the Phase III study by Stenzl et al.13 according to this new definition identified progressive disease in 31/255 patients (12.2%) who had BLC with HAL and 46/261 (17.6%) patients who had WLC at the time of TURBT. Although the comparison between BLC and WLC did not reach statistical significance (p=0.085), time to progression was significantly longer in patients evaluated by BLC with HAL than with WLC (p=0.05).23 The authors propose that adoption of this new definition could allow more patients at risk of NMIBC progression to access BLC.
BLUE LIGHT FLEXIBLE CYSTOSCOPY IN NON-MUSCLE-INVASIVE BLADDER CANCER SURVEILLANCE
The drive to incorporate blue light technology into outpatient surveillance began more than a decade ago, when the feasibility of BLFC in this setting was demonstrated in a small study of 45 patients and confirmed in a prospective Phase II study.24,25 Although BLC was approved across Europe by 2006, uptake of flexible procedures was impeded by poorly developed blue light fibrescopes and insufficient suction to remove debris and urine causing green autofluorescence that obscured the red fluorescence of HAL under blue light. These problems have since been addressed, and currently available videoscopes with a high-power LED light source provide noticeably improved visualisation,26,27 renewing interest in the use of BLFC in the outpatient setting.
Improved detection rates in the surveillance setting could have several potential benefits. Earlier detection of recurrence could allow office management rather than operative management for small tumours and, for high-risk patients, an earlier determination of unresponsiveness to immunotherapy with Bacillus Calmette–Guérin (BCG) could lead to a more timely change in management. In a feasibility study for the use of BLFC and on-site biopsy and fulguration of suspicious lesions as part of routine outpatient management of NMIBC (n=69), it was possible to assess two patients per hour, with <10 minutes required for preparation and instillation of HAL.26 An additional waiting time of 45–60 minutes is required, but patients are free to leave the urology department and come back when cystoscopy is scheduled (patients are instructed not to void in the interim). Of the 14 tumours confirmed by biopsy, 3 were detected by BLC alone (2 Ta, 1 CIS). The majority of tumours could be managed by on-site fulguration; only three cases (21.4%) were referred to the operating room for TURBT with rigid cystoscopy under general anaesthesia.26
Successful implementation of outpatient BLFC was also demonstrated in the first analysis from an ongoing Nordic registry, designed to collect data on the potential benefits of BLC with HAL in patients with suspected NMIBC or for routine cystoscopy after therapy.28 A total of 178 procedures were performed in 136 patients, the majority of which were carried out in clinic; most patients preferred to be treated in this setting rather than by standard TURBT. The beneficial impact of BLFC on lesion detection and management was consistent with the feasibility study, including the possibility for complete in-clinic ablation of tumours. Similarly, no major logistical issues were reported.27,28
PHASE III SURVEILLANCE STUDY
The recent USA approval of BLFC in NMIBC surveillance was based on results from a prospective, multicentre, open-label, Phase III study.8 Eligible patients were required to have a history of multiple, recurrent, or high-grade bladder lesions, and at least 6 weeks since last treatment with intravesical chemotherapy or BCG immunotherapy. The 6-week cut-off was designed to reflect guideline-directed clinical practice as opposed to the more conservative 90 days typically used in earlier studies. Potential bias of within-patient comparisons between white and blue light procedures was minimised by ensuring that randomisation to BLFC took place after the initial white light flexible cystoscopy (WLFC), and toggling back to white light after BLFC was not allowed.
For both procedures, the number, size, and appearance of all suspicious lesions were documented using a bladder map. Resection was not done in-clinic; all patients with suspicious findings were referred to the operating room for standard TURBT under both white and blue light (separate specimens). Final pathology was determined by a consensus panel of pathologists who were blinded to the method of cystoscopy used. The primary efficacy endpoint was the proportion of patients with histologically confirmed malignancy detected by BLFC but not by WLFC. Secondary efficacy endpoints were the proportion of patients with histologically confirmed CIS and additional tumours detected by BLFC in the operating room that were missed by WLFC.8
A total of 304 patients were enrolled in the study, including 202 (66%) with prior high-grade tumours and 184 (61%) who had previously been treated for recurrent disease (mean prior recurrences 1.70±2.03). At prior TURBT, 100 patients (33%) had CIS and 52 (17%) had stage T1 lesions.8 Surveillance identified suspicious lesions in 103 patients, of whom 63 (61%) had bladder cancer confirmed in the operating room. The primary efficacy endpoint was met: BLFC detected recurrent tumours missed by WLFC in 13 patients (20.6%; p<0.0001), whereas WLFC detected only one malignant recurrence that was not seen with the blue light procedure.8 The false-positive rate was 9% for both procedures; this was consistent with previous meta-analyses,29 suggesting that any residual inflammation due to intravesical therapies did not result in a higher number of false positives and supporting a cut-off point of 6 weeks rather than 90 days prior to surveillance cystoscopy.
The detection of additional CIS and malignant lesions with BLFC in the Phase III study was consistent with previously reported rates.11-13,30 Of the 63 patients with confirmed recurrence in the operating room, 26 (41%) were identified as CIS and 9 of these were identified only by BLFC (34.6%; 95% CI: 17.2–55.7; p<0.0001). None of the 9 patients identified by BLFC and not WLFC had positive urine cytology; thus, relying on cytology alone to detect CIS missed by WLFC is inadequate. Consistent with prior Phase III studies, BLFC detected additional malignant lesions (including Ta and T1 tumours) that were missed with WLFC in 29 patients (46.0%; 95% CI: 33.4–59.1; p value not reported).8
SAFETY OF BLUE LIGHT FLEXIBLE CYSTOSCOPY
The Phase III surveillance study provided new insight into the safety of BLFC, particularly regarding repeated administration of HAL (at first cystoscopy and in the operating room for patients with suspicious lesions) and the use of BLFC after intravesical therapy. Prior to the study being published, the USA label for HAL was restricted to single use only. This restriction was lifted based on the Phase III results showing no difference in the adverse events (AE) profile, either at surveillance when comparing prior exposure to HAL or in the 103 patients (33.8%) who had two instillations of HAL during the study.8 The safety of repeat administration of HAL is also supported by results from the prospective BLC registry; 533 patients underwent a total of 641 BLC procedures, with repeat instillations (range: 2–5) performed in 85 patients (16%).20
In the real-world study by Zare et al.,26 no HAL-related AE were reported in 69 patients evaluated by BLFC in clinic. Investigators in the Phase III surveillance study judged that HAL-related AE were experienced by 6 patients following surveillance (2.0%) and 3 patients following the operating room examination (2.9%).8 AE included dysuria, urethral pain, bladder discomfort, erythema, and pruritus following surveillance, and procedural pain and contact dermatitis following repeat use of HAL in the operating room. Importantly, AE rates after the surveillance visit did not seem to be related to either prior use or timing of intravesical therapy. At study entry, prior intravesical BCG or chemotherapy was documented for 82.2% and 37.5% of patients, respectively. The majority of patients (66.7%) received their last dose of intravenous therapy between the 6-week and 90-day cut-offs outlined above.8
PATIENT-REPORTED OUTCOMES AND ACCEPTABILITY
When introducing a new technology, the impact of the procedure must be considered in terms of the patient experience, particularly for invasive procedures where some pain and anxiety may be expected. Both WLFC and BLFC are typically performed with intra-urethral local anaesthetic, but BLFC requires an additional catheterisation to allow for the instillation of HAL and patients must wait for 45–60 minutes after instillation before cystoscopy can be performed. These potentially problematic aspects of BLFC appear to be offset by the advantages of the procedure. Zare et al.26 reported that patients who underwent BLFC in a real-world outpatient setting were more confident in their tumour-free status than if they had received WLFC alone. Although some patients experienced minor discomfort during blue-light-guided biopsy and fulguration, this did not deter them from undergoing the procedure at future surveillance visits.26
These findings are supported by patient-reported outcomes in the Phase III surveillance study.31 Patients were asked to rate their pain and anxiety on three separate occasions (at screening, following first BLFC, and after pathologic diagnosis for patients referred to the operating room with suspicious lesions). At each visit, patients completed the PROMIS (Patient-Reported Outcomes Measurement Information System) Anxiety 4a categorical score32 and Pain Intensity 1a numerical score (scale from 0 [no pain] to 10 [worst pain]).33 Despite the need for catheterisation, pain scores remained low throughout the study. A decrease in anxiety was uniformly observed after surveillance BLFC, with the greatest reduction seen in those with negative pathology. Gender and false positive results did not affect anxiety scores.31
Patients were also asked to assess the value of BLFC surveillance using the ‘Was It Worth It?’ questionnaire.31 More than 90% of patients who underwent BLFC felt it was worthwhile and would recommend the procedure to others. The perceived benefits of BLFC were underlined by the fact that the majority of patients (around 60%) were willing to pay out-of-pocket expenses of US $100 or more to access the procedure. This generally positive perception is consistent with experience showing that patients tend to rate cystoscopic evaluations more highly than other diagnostic tests, such as urinary markers.34
CONSENSUS STATEMENT
The first USA consensus statement on the appropriate use of blue light technology in NMIBC was published in 20146 and has now been updated to include the highest level of evidence for BLC for TURBT and surveillance based on current knowledge. Consensus was based on extensive discussions at the 2018 AUA Meeting by a panel of 17 bladder cancer specialists, 14 of whom had participated in the Phase III BLFC surveillance study.9
There are several scenarios in which BLFC can offer a benefit, especially when combined with in-clinic tumour ablation. There was consensus about the benefits of BLFC at first surveillance cystoscopy at 3 months (when the likelihood of recurrence is highest)16,35 for AUA intermediate and high-risk patients. For high-risk patients, the majority of panellists agreed that BLFC would also be of value at 6, 12, 18, and 24 months, given the high risk of recurrence; about a third supported an additional BLFC evaluation at 9 months. In patients with CIS, BLFC during surveillance was considered to be especially useful for determining lack of response to intravesical BCG. Most panellists felt that surveillance with BLFC was not worthwhile in AUA low-risk patients, since any small low-grade tumour would be unlikely to progress even if missed.9
BLFC also has a potential role in evaluating patients prior to intravesical therapy if there is suspicion of residual disease after TURBT (for example, if performed in a different clinic or without blue light), and to help determine diagnosis in patients with positive cytology but no visible lesions under white light. Tissue biopsy may be performed in-clinic, avoiding the need for referral to the operating room (particularly relevant in patients who are poor surgical candidates). The panel also concluded that, for patients with recurrent low-grade tumours previously diagnosed in the operating room, BLFC may allow better identification and ablation of tumours over white light procedures at the time of in-clinic fulguration and/or biopsy. When considering the use of BLFC in patients with negative cytology and equivocal lesions (erythema) seen on WLC, the panel noted that BLFC may help identify lesions more likely to be malignant than to be white light false-positives.9
The consensus panel also considered the practical and logistical aspects of implementing BLFC in urology departments, including patient selection, scheduling, equipment sterilisation, and training (Box 1). Once the necessary equipment has been acquired, patient selection for surveillance BLFC can start based on a review of pathology after TURBT and upcoming clinic schedules. For example, BLFC can be offered in patients for whom in-clinic cystoscopy and fulguration or biopsy for small tumours is already planned. To ensure a smooth introduction, it is important to remind patients before their first BLFC procedure that they will need to allow at least 60 minutes for instillation of HAL prior to cystoscopy.9

Box 1: Factors to consider when incorporating blue light cystoscopy with hexaminolevulinate into routine practice.
BLC: blue light cystoscopy; HAL: hexaminolevulinate; TURBT: transurethral resection of bladder tumour.
DISCUSSION
BLFC has demonstrated good clinical utility in the outpatient surveillance setting in terms of improved detection of malignant recurrences, especially CIS. The use of BLFC was demonstrated to be safe and feasible, and it had a high level of patient satisfaction. Additional studies are needed to confirm these findings, as well as to identify the optimal timing and frequency of BLFC surveillance and the potential impact on NMIBC progression. The current treatment paradigm for NMIBC will need to be re-evaluated to determine the role of BLFC for surveillance cystoscopy. Additional studies will also be needed to assess the position of intravesical chemo and immunotherapy and in-clinic fulguration within this new paradigm. It is likely that useful insights will be provided by the ongoing Nordic BLFC registry28 and the USA blue light registry, which will expand its scope to include in-clinic evaluations with BLFC.20 Given the multiple strategies for treatment and management of NMIBC, a key challenge will be to determine which patients are likely to derive the most long-term benefit from BLFC.
The need for long-term surveillance of patients with NMIBC following TURBT means that it is critical to evaluate the cost-effectiveness of any changes to the treatment and management of this common and expensive form of cancer.36-38 European studies based on health economic models have demonstrated that the introduction of HAL-guided cystoscopy results in reduced costs over time, in both the diagnostic and surveillance settings.39,40 The economic advantages of replacing inpatient TURBT with outpatient BLFC-guided tumour ablation in patients with low-grade tumours is supported by a recent single-centre analysis from Denmark. In addition to the lower costs of the procedure, the authors also noted the benefits of sparing operating room capacity and the reduced burden on the patient.41,42
There is a clear opportunity here for BLFC to assert a central role in the treatment paradigm, if it can be further demonstrated that adopting the procedure leads to an improvement in cancer detection that results in fewer recurrences or change in management for high-risk patients. Consensus among bladder cancer specialists supports the adoption of BLFC surveillance at the initial 3-month cystoscopy in intermediate and high-risk patients, and at 3 to 6-monthly intervals thereafter for the first 2 years post-TURBT in high-risk patients.9 Further research is needed to clarify the role of BLFC in low and intermediate-risk patients and in other scenarios where it has shown clinical utility.