BACKGROUND
Resting-state EEG (RS-EEG) has emerged as a non-invasive, low-cost tool for Alzheimer’s disease (AD) diagnosis and stratification. This study evaluated whether EEG-based biomarkers can predict amyloid status and clinical progression from mild cognitive impairment (MCI) to AD dementia in patients referred to a third-level memory clinic.1
METHODS
The authors retrospectively analysed 295 patients who were cognitively impaired and underwent a standardised diagnostic work-up, including neuropsychological assessment, cerebrospinal fluid (CSF) biomarker analysis, and 19-channel RS- EEG recording (10–20 system, eyes closed; Table 1). Patients were classified as amyloid-positive (A+; n=184) or amyloid-negative (A-; n=111) based on CSF Aβ42/40 ratio. The MCI subgroup (n=106) was divided into MCI A+ (n=61) and MCI A- (n=45). A total of 39 individuals with MCI A+ were followed over 2 years, and 23 converted to AD dementia.
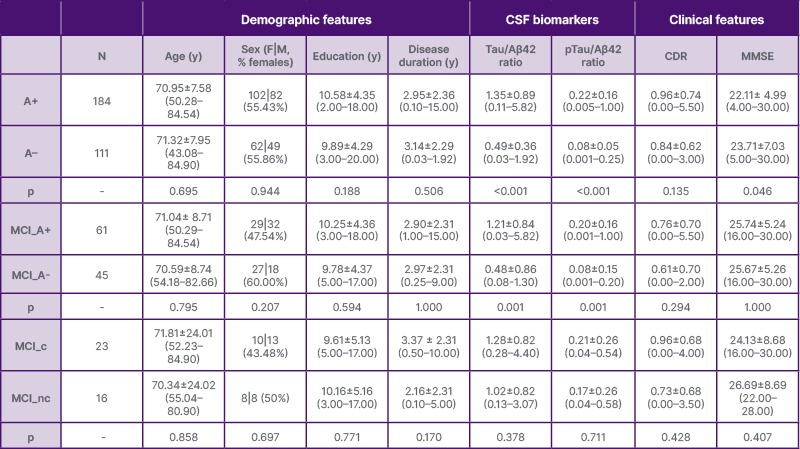
Table 1: Demographic and clinical features of patients stratified according to their amyloid status and conversion status from mild cognitive impairment to dementia.
Values are averages and SDs (range). p values refer to T test models or the Chi-square test. Statistical significance was defined as p<0.05.
A+: amyloid-positive; A-: amyloid-negative; CDR: Clinical Dementia Rating scale; CSF: cerebrospinal fluid; F: females; M: males; MCI: mild cognitive impairment; MCI_A+: amyloid-positive mild cognitive impairment; MCI_A-: amyloid-negative mild cognitive impairment; MCI_c: mild cognitive impairment converters; MCI_nc: mild cognitive impairment non-converters; MMSE: Mini-Mental Status Exam; N: number; pTau: phosphorylated Tau; y: years.
EEG preprocessing included bandpass filtering (1–45 Hz), independent component analysis-based artefact removal, and re-referencing to the average. Source-space analysis was performed using standardised low-resolution brain electromagnetic tomography, constrained to two networks of interest: the default mode network (DMN), typically affected in AD, and the salience network (SN), more often impaired in non-AD conditions. These networks were defined through a seed-based resting-state functional MRI analysis conducted in an independent cohort of healthy young adults, using canonical seeds placed in the posterior cingulate cortex (DMN) and anterior cingulate (SN). The resulting network maps were coregistered in Montreal Neurological Institute (MNI) space and parcellated into regions of interest. EEG source activity was then estimated voxel-wise and averaged across each region of interest to extract measures of current source density (CSD) and pairwise linear lagged connectivity (LLC), across δ (1–3.5 Hz), θ (4–7.5 Hz), α1 (8–10 Hz), α2 (10.5–12 Hz), and β (12–30 Hz) bands.
RESULTS
Patients who were A+ showed a general slowing of EEG cortical activity when compared to A- in both DMN and SN. Patients with MCI A+ showed significantly increased θ CSD and LLC in both networks, with peak differences in posterior DMN regions, consistent with early network-level hyperconnectivity. Among subjects with MCI A+, converters exhibited reduced α1 CSD in the DMN and decreased α LLC across both DMN and SN. No significant changes emerged in the β band.
Support vector machine classifiers using significant EEG features were trained to predict amyloid status (at both whole group and MCI group levels) and MCI conversion. Feature selection was guided by Shapley Additive Explanations, identifying occipital θ CSD and frontal α1 LLC as most predictive. The best-performing support vector machine models achieved around 60% balanced accuracy in all classifications, with robust results across 5-fold cross-validation.
CONCLUSION
This study underscores that AD causes early disruptions in cortical electrical activity, detectable through resting-state EEG. θ current source density and network connectivity emerged as early markers of amyloid-related pathology, while α connectivity remained relatively preserved in early stages and declined in patients progressing to dementia. These findings reflect a trajectory from early excitatory θ alterations to α disconnection, marking synaptic dysfunction and neurodegeneration.2-4 Machine learning models offer promising avenues for early detection and risk stratification.5 Notably, the DMN was more consistently impaired than the salience network, highlighting its selective vulnerability in AD.2,3
RS-EEG may support scalable, non-invasive screening strategies in memory clinics, especially where access to amyloid PET or CSF biomarkers is limited. Future directions include longitudinal validation, integration with plasma biomarkers, and multimodal frameworks for non-invasive prediction of disease progression.







