Abstract
Objective: In 2001, the introduction of non-resectional lung volume reduction surgery (LVRS) enabled surgery under non-intubated anaesthesia. This study compares this combined technique to a group of patients with a similar disorder who refused non-intubated anaesthesia.
Methods: Between January 2001 and October 2015, 108 patients with severe emphysema underwent non-resectional LVRS under non-intubated anaesthesia. During the same period, another 15 patients scheduled for LVRS refused non-intubated surgery and underwent the same procedure under traditional intubated modality. Respiratory and functional parameters were evaluated. Time to residual volume recurrence and overall survival were analysed with the Kaplan–Meier method.
Results: Thirteen cases (12%) required intubation due to tenacious pleuropulmonary adhesions (n=7) or intolerance (n=6). Compared with the intubated group, the non-intubated group were found to have significantly better results in post-operative partial pressure of carbon dioxide in arterial blood (PaCO2) (45±8 versus 52±8 mmHg; p=0.04), global operative time (41±24 versus 72±31 minutes; p=0.01), non-fatal complication rate (13.6% versus 33.3%; p=0.029), and especially postoperative pneumonia rate (3.1% versus 33.3%; p=0.004); patient satisfaction for anaesthesia was also improved in the non-intubated group (3.6±1.2 versus 2.8±1.7; p=0.03). Mean air leakage (5.3±3.5 versus 6.1±4.6 days), hospital stay (6.3±4.8 versus 8.0±6.1 days), and 90 days postoperative mortality rate (1.0% versus 6.6%) were lower, yet not significantly, in the non-intubated cohort. All mean respiratory and symptomatic parameters significantly improved in both groups, with no intergroup significant difference, and persisted for 4 years after surgery. Mean follow-up for the non-intubated group was 78±30 months. Analysis of time to residual volume recurrence and overall survival showed no statistically significant intergroup difference.
Conclusion: Non-intubated, non-resectional LVRS presents a 90-day postoperative non-fatal complication rate and patient satisfaction for non-intubated anaesthesia that are significantly better than intubated procedures. The long-term outcomes were similar between both groups.
INTRODUCTION
Lung volume reduction surgery (LVRS) has proved an effective treatment in palliating emphysematous symptoms in selected patients.1 This procedure was primarily performed as a trans-sternal anatomical bilateral resection on the most severely emphysematous target area.2 Subsequently, resectional LVRS was proposed in either a unilateral or bilateral (one stage or two stages, respectively) video-assisted thoracoscopic (VATS) approach, giving preference to the most damaged side, which similarly achieved satisfying results.3 In the same period, fold plications without resection, i.e., non-resectional, were carried out for the treatment of bullous emphysema through either thoracotomy4 or VATS5 approaches.
On the basis of the two procedures aforementioned, Mineo et al.6 successfully proposed a variant of the original LVRS technique, which consisted of the simple non-resectional plication of target areas. This procedure proved to be suitable for conscious patients, without the need for a single-lung ventilation, although the procedure was equally effective as resectional LVRS.7 The operation was primarily conducted under thoracic epidural anaesthesia8 and via a multiportal approach;6 however, more recently a uniportal access under intercostal local block was adopted.7
In this observational study, the outcomes of a cohort of patients undergoing non-intubated, non-resectional LVRS are reviewed and compared with the long-term outcomes of similar patients who refused non-intubated operation.
MATERIALS AND METHODS
Patient Setting and Population
Between January 2001 and October 2015, a total of 108 consecutive patients with moderate-to- severe emphysema underwent non-resectional LVRS under non-intubated anaesthesia. In 77 patients, the procedure was accomplished through a conventional multiport VATS approach under epidural anaesthesia. The final 31 patients were operated on using a uniportal technique under intercostal block. The Tor Vergata University’s (Rome, Italy) Institutional Review Board allowed the retrieval of all patient data regarding patient follow-up. The retrospective study was approved by the ethics committee of Tor Vergata University. During the same period, another 15 patients scheduled for LVRS refused non-intubated surgery and therefore underwent the same non-resectional procedure under general anaesthesia with one-lung ventilation; these patients were subsequently used as the control group.
All patients gave written informed consent after reading a written explanation of the main characteristics and theoretical advantages and disadvantages of non-resectional LVRS performed either by sole epidural anaesthesia, intercostal nerve block, or through general anaesthesia and one-lung ventilation. The form stressed specifically that during an awake operation, some surgical manoeuvres might be found more technically demanding, and that the procedure might be less comfortably tolerated due to risks of hypercapnia and panic attacks; the immediate postoperative course, however, was expected to be smoother than after general anaesthesia, due to the lack of weaning-related adverse effects. Conversely, general anaesthesia may have allowed achievement of an immobile operative field, and avoided the risk of panic attacks, although a longer stay in the recovery room and early postoperative respiratory discomfort were likely to be more common.
Preoperative Assessment
All patients underwent radiologic studies, including digital inspiratory and expiratory chest X-ray, and high-resolution chest computed tomography (CT). Dynamic and static pulmonary function tests were performed before and after inhalation with two puffs of aerosolised β2-agonists. Exercise tolerance was tested by both a 6-minute walking test and using maximum increments on a treadmill. The degree of dyspnoea was scored according to the modified Medical Research Council Score.9 Quality of life was quantified using the St. George’s Respiratory Questionnaire.10
Indications
Indications towards LVRS originated from panel discussions among surgeons, pulmonologists, anaesthesiologists, intensive care specialists, physiotherapists, and psychologists. The main inclusion criteria included severe respiratory impairment characterised by the triad of bronchial obstruction, defined as when forced expiratory volume in 1 second is ≤40% than predicted, despite a bronchodilator; hyperinflation, when residual volume is ≥180% than predicted; and upper lobe targeted disease with compressed adjacent parenchyma. These patients must quit smoking and show determination and the capability to sustain a structured preoperative respiratory rehabilitation programme. With the introduction of non-resectional, non-intubated procedures, both old age and comorbidities have progressively become less strict exclusion criteria.
In addition, other exclusion criteria were clinical obesity (BMI ≥30 kg/m2), imaging or clinical suspect for an obliterated pleural cavity, refusal of an awake procedure and an extreme anxious attitude, haemodynamic instability, inability to co-operate, and any contraindication to the planned regional anaesthetic technique (e.g., coagulopathy). In the case of asymmetric emphysema, the procedure was initially only performed on the most damaged side, subsequently determining the clinical, functional, and anatomical postoperative evolution of the opposite, less damaged side. Conversely, in the presence of a symmetric distribution of disease and damage within the lungs, LVRS was performed as a staged bilateral procedure with an interval of no longer than 4–6 weeks between surgeries.
Anaesthesia
Epidural anaesthesia
The anaesthetic was administrated through a thoracic epidural catheter inserted at T4 after premedication of 7.5 mg midazolam. In the operating room, patients received a continuous infusion of 0.5% ropivacaine and sufentanil 1.66 μg/mL into the epidural space. In some instances, a topical vagal blockade was also performed. The anaesthetic was administrated using multiportal VATS.
Intercostal block anaesthesia
After insertion of venous and radial artery catheters, an aerosolised 5 mL solution of 2% lidocaine hydrochloride was administered for 5 minutes to avoid a cough reflex. The intercostal block was accomplished by a local injection of a 20–30 mL solution of 2% lidocaine and 7.5% ropivacaine to achieve a rapid onset with a long-lasting analgesic effect. The inoculation was performed along the surface area selected for uniportal VATS, and included subcutaneous layers, intercostal nerves, and parietal pleura. The grade of local anaesthesia was always adequate. In a few cases, benzodiazepine (midazolam 0.03–0.1 mg/kg) or opioids (remifentanil 15 µg/kg/min) were intravenously supplemented during lung manipulation. The employment of a bispectral index to monitor the level of sedation during the operation allowed the regulation of the sedation level through a real-time measurement.
General anaesthesia
Patients undergoing non-resectional LVRS through general anaesthesia received a thoracic epidural catheter insert between T5 and T8, and a continuous infusion of ropivacaine. General anaesthesia was induced with intravenous propofol (1.5–2.0 mg/kg), fentanyl (0.1 mg), and vecuronium (0.1 mg/kg), and maintained using a continuous infusion of these drugs.
Surgical Technique
The operation was performed in full lateral decubitus with multiportal VATS associated with epidural anaesthesia or in the last period with uniportal VATS associated with intercostal block. When the operations were performed with multiportal VATS, four flexible thoracoscopic trocars were used; one for the operative thoracoscope, usually placed in the sixth intercostal space along the midaxillary line, and the others placed in the third and fifth intercostal space along anterior axillary lines, and in the fourth intercostal space along the posterior axillary line. When the operations were performed with uniportal VATS, the single port of 30–40 mm was carried out along the space judged to be the most suitable to reach the foreseen area. However, most of the time the port was placed in the fourth intercostal space because the upper lobe of the lung is the area most affected. The operation aimed to reduce 20–30% of the lung volume by removing functionally ineffective hyperinflated lung tissue through plication without resection.
A 10 mm camera, angled at 30°, was used to facilitate oblique vision of the lung in spite of spontaneous ventilation. The most emphysematous target areas were visualised and pushed down with a cotton swab, while anelastic lung edges were grasped with ring forceps to create two parallel ridges of non-functional, redundant tissue. After this, a 45 mm ‘no knife’ endostapler with 3.5 mm cartridges was applied on the placated lung region. Following this, the two folds were distally sutured apart from each other by a supplementary no knife stapler in order to buttress the plication structure and protect the previous stapling lines. The plication cyanoacrylate application (Glubran 2®, GEM SRL, Viareggio, Italy) was used on the lung tissue to minimise the risk of air leak.
At the end of the procedure, one 28 Ch chest tube was collocated through the posterior end of the incision. The insertion of one transintercostal suture that significantly reduced the onset of subcutaneous emphysema was found to be useful. Muscle sutures were tightened after asking the patient to breathe deeply or cough to achieve maximal lung re-expansion. Unilateral LVRS was performed in patients with distinct radiologic evidence of between-lung heterogeneity of emphysema (asymmetric emphysema), while a symmetric distribution of disease within the lungs was considered an indication for a staged bilateral treatment (no longer than 4–6 weeks from the first procedure).
Follow-Up
Respiratory and functional results were initially evaluated every 6 months for the first 2 years, and discussed within the aforementioned panel group. Thereafter, control groups were assessed yearly. Further LVRS was reconsidered in cases of functional or spirometric decline to the preoperative status. In such instances, contralateral or ipsilateral re-do procedures were proposed, with CT showing evident lung destruction or hyperinflation in a defined targeted area with relative conservation of the underlying parenchyma.
Statistical Evaluation
Descriptive statistics were presented as mean ± standard deviation, while post-treatment changes were indicated as the mean percentage of the baseline value. Due to the relatively small sample size, non-parametric tests for paired and unpaired comparisons were used (Wilcoxon rank-sum test and Mann–Whitney U test, respectively). Analysis was conducted using SPSS® 19.0 version (SPSS Inc., Chicago, Illinois, USA). The significance was set at p<0.05. Survivals and time-to-event evaluations were performed by the Kaplan–Meier method.11 The day of operation was used as the starting point and the day of residual volume returned equal to baseline value as the endpoint. The significance test was assessed according to the Mantel log-rank test.
RESULTS
The two groups (non-intubated versus intubated) were homogeneous for anagraphic and clinical data. The non-intubated group showed a higher average age (65±6.1 versus 60±9.6 years). It was demonstrated that the overall stay in operatory theatre for the non-intubated group was significantly shorter than the intubated group (41±24 minutes versus 72±31 minutes; p=0.01). Global conversion rate to general anaesthesia was 12.0% (13 of 108), and this was equally due to surgical reasons, such as tenacious adherences (n=7) and intolerance to sustain non-intubated anaesthesia (n=6).
Early Outcomes
Early outcomes are documented in Table 1. It was noted that all measured oxygenation parameters were similar between groups, except arterial pressure of carbon dioxide, measured 1 hour postoperatively, which was significantly lower in the non-intubated group (45±8 versus 52±8 mmHg; p=0.04). There was no perioperative mortality in either groups. Compared to the intubated group, 90 days postoperative mortality was lower in the non-intubated group, with 1.0% versus 6.6%. Only one death was experienced within 90 days due to acute pneumonia. The non- fatal complications rate was significantly lower than that experienced after intubated LVRS (13.6% versus 33.3%; p=0.029). Among these, only three patients developed early postoperative pneumonia, compared with three patients in the intubated group (p=0.004). Persistent air leakage (>7 days) was experienced in 24 patients (25.2%); only three of which required restapling of the suture.
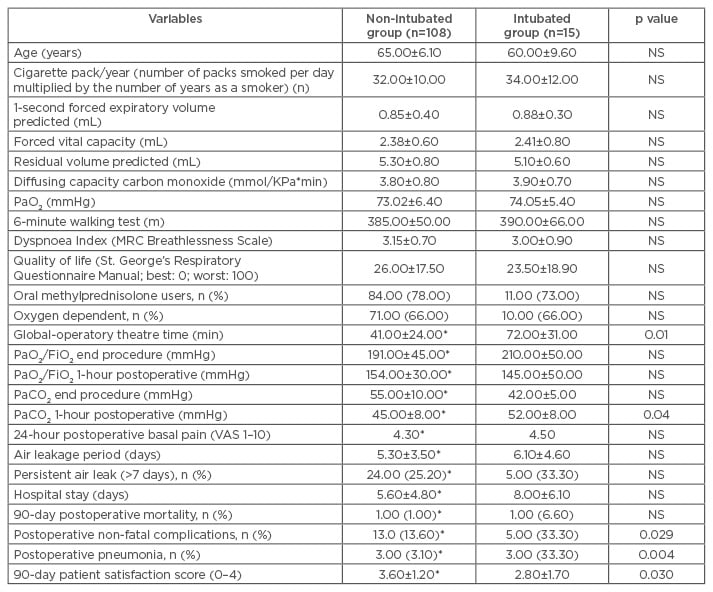
Table 1: Baseline, intraoperative, and immediate postoperative comparisons between study groups.
*Limited to 95 patients, after excluding those converted to open access or intubated anaesthesia.
FiO2: fractional inspired oxygen; MRC: Medical Research Council; NS: not significant; PaCO2: partial pressure of carbon dioxide; PaO2: partial pressure of oxygen; PaO2/FiO2: ratio of arterial oxygen partial pressure to fractional inspired oxygen; VAS: visual analogue scale.
Mean air leakage period (5.3±3.5 versus 6.1±4.6 days) and hospital stay (6.3±4.8 versus 8.0±6.1 days) were shorter in the non-intubated group; however, these results are not significantly different (Table 1). Patient satisfaction for anaesthesia 90 days after surgery (0=minimal satisfaction, 4=maximal satisfaction) was significantly greater in the non-intubated group (3.6±1.2 versus 2.8±1.7; p=0.03) (Table 1).
Long-Term Outcomes
Mean follow-up of the non-intubated group was 78±30 months and 85±13 months for the intubated group. No patients (except those who died) were lost at follow-up over the first 4 years after procedure. Thereafter, 11 patients in the non-intubated group and 2 patients in the intubated group were lost. Mean values of all respiratory and symptomatic parameters significantly improved in both groups, with no intergroup significant difference. All the mean values persisted better than the preoperative baseline for 4 years after surgery (Table 2). In 71 patients, LVRS was repeated on the other side of the body after a mean period of 36±18 months. Namely, residual volume persisted higher than preoperative values for more than 36 months in 24 patients. This evolution was not significantly different from that documented after the intubated procedure (Figure 1A and 1B). Analysis of long-term survival showed no statistically significant difference between non-intubated and intubated groups, in terms of both time to residual recurrence (p=0.4) (Figure 1A) and overall survival (p=0.7) (Figure 1B).
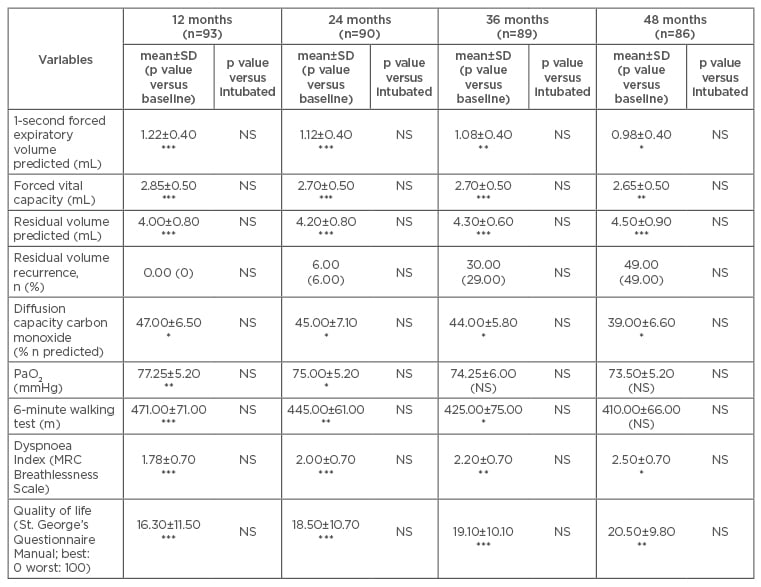
Table 2: Postoperative mean ± standard deviation values and p values compared to baseline value.
*p<0.05; **p<0.01; ***p<0.001 and control group.
MRC: Medical Research Council; NS: not significant; PaO2: partial pressure of oxygen; SD: standard deviation.
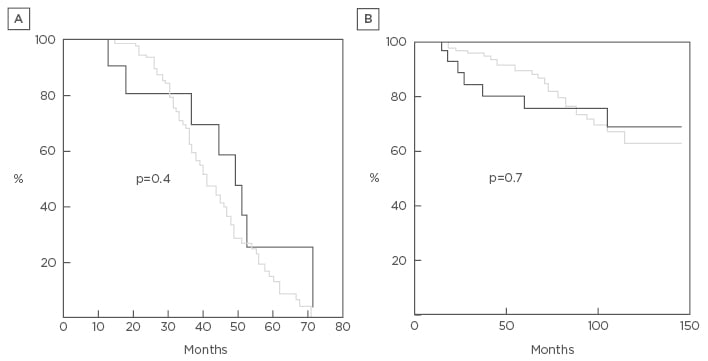
Figure 1: Kaplan–Meier curve results.
A) Time to residual volume recurrence between non-intubated (grey) and intubated (black) lung volume reduction surgery; B) overall survival between non-intubated (grey) and intubated (black) lung volume reduction surgery. The curves are similar throughout the study period. No statistically significant differences were found at the log-rank test.
DISCUSSION
Pulmonary emphysema is a very common disease and is still characterised by a significant mortality rate.12 LVRS proved effective in improving symptoms for a variable and yet significant period in patients with upper lobe predominant emphysema.13-17 Pulmonary hyperinflation causes loss of lung elastic recoil and an unfavourable length–tension relationship of the diaphragm, limiting its capacity to produce effective airflow.18 Accessory respiratory muscles are activated and work against a high load, which is worsened by loss of muscle efficiency.19 The physiological basis of the procedure lies on the reduction of hyperinflation, and the restoration of normal parenchyma elasticity with improvement of respiratory muscle dynamics, increase in oxygen availability, and less energy expenditure.20
All these steps can be surgically accomplished by resecting the most damaged lung area. Nevertheless, according to previous experiences, even the non-resectional lung plication technique may achieve similar results.6 The procedure proved simpler and quicker than resectional LVRS and avoided interruption of bronchovascular structures. These evident advantages were weakened by the significant postoperative impairment due to general anaesthesia with one-lung ventilation, which had still remained significantly elevated, hindering the acceptance of the treatment among both patients and physicians.21
In the present study, non-intubated LVRS was safely and easily performed under thoracic epidural anaesthesia7 or local anaesthesia.8 This procedure was promptly accepted by the patients, while also meeting the compliance of the deputed physician and referred pulmonologists. This renewed confidence implies that many patients are referred to the surgeon and operated on earlier than in other centres. The evolution of early postoperative gas exchanges documents that hypoxia and hypercapnia worsening during non-intubated anaesthesia can be rapidly resolved. Avoidance of general anaesthesia, reflected in satisfactory perioperative respiratory performance, allowed a more rapid recovery with shorter hospitalisation, and immediate return to many day-to-day activities, including drinking, eating, and walking. As a matter of fact, this study experienced a significantly lower 90-day postoperative non-fatal complications rate, especially with regard to postoperative pneumonia incidence. This is a major benefit of non-intubated procedures because they allow constant ventilation during the operation with better postoperative ventilation.6 As a consequence, non-intubated techniques allow shorter hospital stays without affecting incremental improvements incurred in clinical and respiratory function measurements.
No differences were found in long-term outcomes between non-intubated and intubated anaesthesia. In more than three-quarters of the patients from both groups, the residual volume persisted below the preoperative value for >36 months. The majority of the early recurrences of the residual volume were due to the small postoperative increase in the value, despite the postoperative amelioration of the flows and the subjective symptomatic improvement. In another subset of patients, the rapid worsening of residual volume value was unpredictable, which could potentially signify a different genetic base.
In the final period of the study, a uniportal approach was initiated that was found to be efficacious and safe.8 These early results must be evaluated after a longer follow-up to provide more reliable data. The authors acknowledge limitations of the study, which are partially mitigated by the observational nature of the study. The major limitation of the study is the retrospective non-randomised nature of the investigation, but this evident flaw can be counterbalanced by the consistent sample size collected in one single institution. The results suggest that non-intubated, non-resectional LVRS is safe and reliable. Compared to intubated similar procedures, this technique can achieve a significantly shorter global operative time, lower rate of 90-day postoperative non-fatal complication rate, and improved patient satisfaction for anaesthesia without affecting long-term outcomes.








