Abstract
Over the years, various allergen inhalation challenge models have been developed to study the pathophysiology and pharmacology of allergen-induced asthma. Each allergen challenge method possesses unique benefits and disadvantages. The classic allergen challenge model is useful for assessing the efficacy of new treatments but does not reflect real-world repeated exposure and excludes approximately 50% of allergic asthmatics (i.e. those who do not exhibit a late asthmatic response). The early response model, while also artificial, is less time-consuming and allows for the generation of dose-response data but does not assess the late response or related sequelae. The repeated low-dose allergen model was developed with the purpose of mimicking natural exposure for induction of airway inflammation and airway hyperresponsiveness. However, this method does not consistently produce airway inflammation and is less practical to perform due to the number of study visits required. The segmental allergen model is the only one to allow direct sampling of airway secretions for airway inflammation studies, but it is highly invasive and requires special training and equipment. Attempts have been made to establish a repeated high-dose allergen model for the assessment of drug effects on symptoms and rescue medication use, but participant safety remains a concern and it is also less practical than the classic method. The most difficult allergen model to perform is the natural exposure method, for which standardisation may not be possible given the number of environmental factors that must be controlled or measured. Modifications to these allergen models could improve their clinical relevance and identify their specific, tailored applications in pharmaceutical research of allergic asthma.
INTRODUCTION
Asthma is a common chronic respiratory condition that physically, mentally, and financially affects approximately 300 million people worldwide.1 Over the last 30 years, significant research findings have transformed our knowledge of the causes, pathophysiology, symptoms, and treatments for asthma, but much has yet to be investigated or further elucidated. In atopic asthma, mechanisms and novel respiratory medications are studied through inhaled allergen challenge testing. This communication will briefly review the history of the allergen challenge and the various experimental models that have been used for allergen challenge testing. Recommendations for minor changes in the commonly used classic model will also be made.
EVOLUTION OF THE ALLERGEN CHALLENGE
The allergen challenge model has undergone several changes in the last century as a result of advancements made in technology and research. Early allergen challenges measured fluctuations in vital capacity to observe the allergic response and any treatment or hyposensitisation effects.2 The forced expiratory volume in 1 second (FEV1), invented by Tiffeneau in 1947,3 has since become the standard lung function parameter assessed; the FEV1 is significantly more sensitive and does not require the induction of a high, likely uncomfortable, and potentially unsafe level of bronchoconstriction.4
Mid-20th century allergen challenge testing entailed administering nebulised allergen extracts via a bell spirometer/air pump/nebuliser circuit, through which dose delivery depended on inhalation time.2 Such experiments identified multiple key factors involved in the allergic response that are still clinically relevant today. The primary endpoint of these historic studies was the change in the immediate or early asthmatic response (EAR).5 In the 1950s, studies identified the late asthmatic response (LAR),5-7 which peaks several hours after allergen exposure.
Originally, allergen challenge testing was used to diagnose allergic sensitivities and possibly hyposensitise patients to their allergies.5 Its use for assessing and comparing drug effects was relatively new in the 1950s,2 but has since become its primary application. It was subsequently discovered that airway hyperresponsiveness (AHR) to non-specific or direct-acting stimuli (e.g. methacholine) increased with allergen exposure and was associated with the LAR.8,9 Current allergen challenges include baseline and post-allergen measurements of AHR, typically through methacholine challenge testing (MCT). Differences in the levels of inflammatory factors in the bronchoalveolar lavage and sputum samples of asthmatics post-allergen were also observed in the late 20th century.10,11 Most modern pharmaceutical allergen challenge protocols require dual responders and investigate therapeutic efficacy, focussing on the LAR and airway inflammation.12 A number of challenge models are used (Table 1).
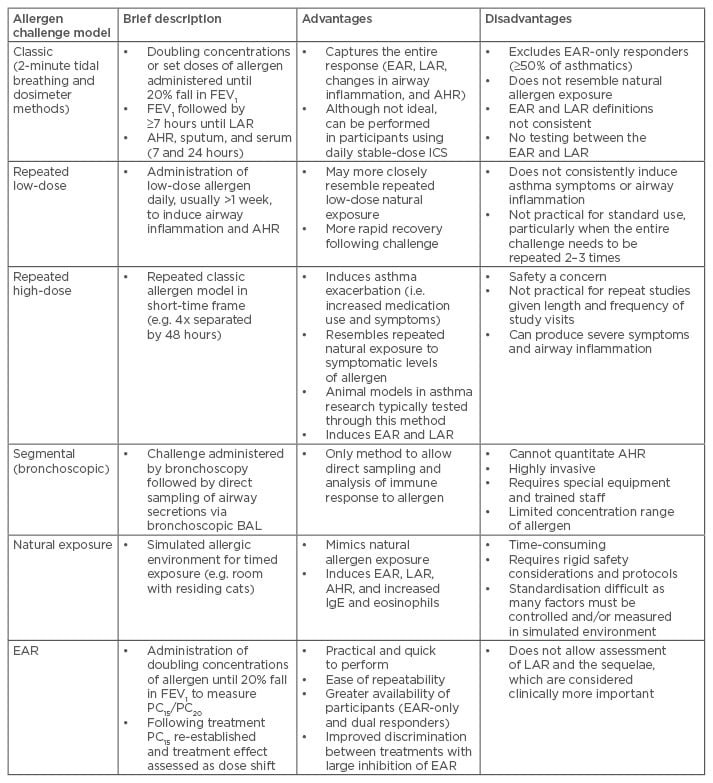
Table 1: Allergen challenge models and their advantages and disadvantages.
AHR: airway hyperresponsiveness; BAL: bronchoalveolar lavage; EAR: early asthmatic response;
FEV1 : forced expiratory volume in 1 second; ICS: inhaled corticosteroids; IgE: immunoglobulin E; LAR: late asthmatic response; PC15/PC20, provocative concentration causing a 15/20% fall in FEV1.
CLASSIC ALLERGEN CHALLENGE MODEL
In comparison to historical methods, the classic allergen challenge model involves more precise methodologies.13 When using the 2-minute tidal breathing method, a safe starting allergen concentration is deduced through MCT and skin prick test results, from which the provocative concentration of allergen causing a 20% fall in FEV1 (PC20) can be predicted.14 The starting concentration is 3–4 doubling concentrations less than the predicted allergen PC20 and is held constant for each allergen challenge for the duration of the study.15 Doubling concentrations of allergen are administered over 2 minutes of tidal breathing and a single FEV1 measurement is obtained 10 minutes post inhalation. Administration of the next concentration begins 12 minutes after the start of the previous, and this process repeats until a 20% fall in FEV1, relative to the highest baseline FEV1, is achieved.12
When using the dosimeter method, a set pattern of allergen doses is administered via a dosimeter.16–18 FEV1 measurements are recorded 10 minutes post allergen inhalation,16,17 or at 5, 10, and 15 minutes post-dose before the next inhalation.18 Testing is typically continued until the FEV1 falls by at least 20% from baseline and a cumulative provocative dose (PD20) is obtained.16–18 The same inhalation pattern or a single inhalation challenge using only the cumulative dose of allergen is then used for subsequent challenges.
For both methods, after allergen inhalation is complete, FEV1 measurements are captured at set time points up to 7 hours (or longer) to follow the potential development of an LAR. This model is commonly used to investigate drug effects on the LAR, usually as changes in maximal decrease in FEV1 and/or area under the FEV1 curve, provided the same dose of allergen has been administered before and after the intervention under investigation. The model also allows for secondary investigations of changes to the EAR, as well as allergen-induced late sequelae, including increases in airway inflammation10,11 and in AHR to methacholine.8,10
Despite standard use in pharmaceutical research, classic allergen challenge methods are not flawless. Current study protocols are designed to exclude non-LAR responders; this is problematic, because ≥50% of allergic asthmatics do not develop an LAR, possibly due to the allergen type and/or dose administered.12,19 For example, multiple studies have reported that asthmatics allergic to house dust mites are significantly more likely to develop an LAR, which is often also of higher magnitude compared to other allergic triggers.17,20,21 Nonetheless, the exclusion of isolated EAR responders calls into question the generalisability of study findings; however, this model is highly appropriate for studies strictly focused on the LAR. Additional concerns with this model are that the dose delivered may not resemble environmental allergy exposure19 and that there is a lack of standardisation when defining the EAR and the LAR. The EAR has been defined as a 20% fall in FEV1 within 5–30 minutes,19,22,23 10–30 minutes,24,25 0–1 hour,26 0–2 hours,27,28 or 0–3 hours post-allergen;29 the LAR has been defined as a minimum 15% fall in FEV1 2–8 hours,19 3–7 hours,27,29,30 3–8 hours,28 4–10 hours,24,25 2.5–10 hours,18 3–10 hours,26 or 3–24 hours post-allergen.31 Studies of this nature have also been limited in that researchers have not performed experiments in the period between the EAR and LAR because of the risk of influencing the development of their primary study endpoint, the LAR.
REPEATED LOW-DOSE ALLERGEN CHALLENGE MODEL
Some researchers have focussed on establishing a reproducible approach for performing repeated low-dose allergen challenges. Their rationale is that many asthmatics are regularly exposed to a low level of their allergen, which increases airway inflammation and AHR.30,32 This model entails the administration of the allergen PC5 pre-determined through a screening classic allergen challenge.30,32,33
Sulakvelidze et al.32 designed and tested administering a dose of allergen in the morning for 5 consecutive days, with MCT and sputum induction performed at screening and on the afternoon of Days 1, 3, and 5 of the trial. Changes in symptomology and rescue medication use were assessed through questionnaires. A slight drop in baseline FEV1 was observed, but significant increases in airway eosinophil and interleukin (IL)-5 levels, AHR, asthma symptoms, and rescue therapy use occurred. All effects subsided within 3 days, which is a more rapid recovery than following a classic allergen challenge. De Kluijver et al.33 completed a similar study but dosing was performed for 10 business days over 2 consecutive weeks. Overall, it was found that this protocol produced a significant increase in airway inflammation (i.e. exhaled nitrous oxide, sputum eosinophils, and sputum IL-5) without increasing symptomology or AHR.
Palmqvist et al.30 explored the effects of repeated low-dose allergen exposure on the allergic response to a high-dose challenge. Participants underwent 7 consecutive days of repeated low-dose cat allergen challenge, followed 48 hours later by a single high-dose challenge (i.e. cumulative dose from screening) to determine the effect, if any, on the LAR. Repeated low-dose allergen exposure resulted in a small but significant reduction in the high-dose challenge LAR (30%), even though airway responsiveness to methacholine and the number of sputum eosinophils had significantly increased following the final low dose challenge performed 2 days earlier.
Despite the perceived immunotherapeutic effect, the low-dose approach has several disadvantages. It does not consistently induce asthma symptoms,18 which precludes the concomitant investigation of treatment effects on the allergic response and on patient symptomology. This method also does not consistently induce elevated airway inflammation,18 making it, in its current form, less reproducible than the classic method. Lastly, it is not practical for standard use, as it requires several more study visits than the standard high-dose method.
REPEATED HIGH-DOSE ALLERGEN CHALLENGE MODEL
The repeated high-dose model is meant to mimic environmental exposure to a significant level of allergen, thereby inducing an asthma exacerbation, sustained airway eosinophilic inflammation, and structural remodelling.18 The other testing models do not resemble the situation where patients are repeatedly exposed to symptomatic levels of their allergen. Additional rationale for this method is that animal models of asthma used in early pharmaceutical studies are often tested through repeated high-dose allergen challenges,18 and so following a similar procedure in humans would allow for more appropriate transferring of findings between species.
Grainge and Howarth18 investigated a repeated high-dose allergen challenge protocol that entailed three classic allergen challenges at 48-hour intervals. In case sensitisation was to develop, the last two allergen challenges were performed starting with the dose equivalent to half the PD15 of the first challenge. It was subsequently found that no priming or desensitisation occurred, and the dose of allergen administered across the three challenges was equivalent. The researchers also observed a significant increase in the frequency of symptoms and rescue therapy use, while the baseline FEV1, EAR, and LAR values did not significantly change over the course of the trial. Unfortunately, this study did not investigate changes in AHR or airway inflammation.
Only this allergen model seems to produce significant increases in the frequency of symptoms and rescue medication use; however, safety remains a primary concern with this approach and its feasibility in the research setting is questionable, as it requires multiple long allergen challenges in a short timeframe. Another limitation is interpretation of changes in PD15 across a treatment period. It is possible that incidental exposures to stimuli altering responsiveness to allergens may have unknowingly occurred during the interval (i.e. 48 hours) between challenges. Others have assessed shortening the interval between repeated high-dose challenges, which produced severe symptoms and airway inflammation, raising safety and ethical concerns for routine use of this type of protocol.16 Altogether, more research is needed on this approach to allergen testing before it can be used as a common laboratory technique.
SEGMENTAL ALLERGEN CHALLENGE MODEL
As the most invasive allergen challenge method, the segmental allergen model, also known as the local allergen challenge, is particularly useful for investigating the pathogenesis of asthma at the cellular level.34 It allows for direct sampling of airway secretions through bronchoalveolar lavage and so the immune response to allergen can be more thoroughly studied through analysis of specific cells, mediators, and cytokines in bronchoalveolar lavage fluid.16,35
Metzger et al.35 assessed the allergic response in asthmatics through a local allergen challenge. Participants first underwent MCT and a classic allergen challenge to establish the baseline AHR and allergic response. They then underwent a control bronchoalveolar lavage and bronchoscopy prior to the local allergen challenge. Increasing concentrations of allergen aliquots were administered via the bronchoscope until there was a visible airway response to the allergen, at which point airway secretions were collected for analysis. Participants returned 2–4 days later for one last bronchoalveolar lavage. The airway secretions were then analysed for the level of macrophages, lymphocytes, neutrophils, basophils, mast cells, and eosinophils present. It was not possible, however, to quantify the level of airway narrowing produced.
Although this model is unique in its ability to directly observe changes in the airway at the cellular level, it is not practical to perform as it is highly invasive and requires specially trained staff and specialised equipment.34 It also does not allow for the administration of relatively high concentrations of allergen locally, as the endpoint is the visual change in airway response, which may occur at lower concentrations of allergen. As such, the segmental allergen challenge testing is not often used and is only implemented in a narrow research context.
NATURAL EXPOSURE ALLERGEN CHALLENGE MODEL
A natural exposure allergen challenge has also been attempted and this should most closely resemble true environmental exposure. However, several problems arose with this methodology; many factors must be rigorously controlled and can be difficult to measure, such as the dose and particle sizes of allergen received, the setting of exposure, the duration of the challenge, and the target endpoint.31
Arvidsson et al.31 completed a crossover study to compare allergen challenge results from the classic model and a natural exposure method. The ‘natural’ allergic environment consisted of a sitting room where cats had lived for ≥10 years. The approximate allergen dose was calculated through analyses of air samples and settled dust. During the natural exposure challenge, participants spent ≤3 hours in the unventilated room and were exposed to live cats for approximately 20% of the provocation time. FEV1 measurements were captured every 15 minutes until an FEV1 fall of 20% was achieved. After the natural exposure challenge, the FEV1 was measured hourly until bedtime and at 24 hours post-allergen. It was found that with the natural exposure method, 60% of participants developed an EAR and 47% developed an LAR. Overall, the two allergen models did not produce differences in sputum content, specific immunoglobulin E (IgE) levels, or sputum eosinophils, and 65% of the sample produced similar EAR and LAR responses to both methods. It was also observed that the results with the two models became significantly more comparable when participants who experienced a 10–15% fall in FEV1 in the late phase were included as late responders.
Unfortunately, the natural exposure model is difficult to perform, is time-consuming, and requires rigid safety considerations and protocols. As such, it remains the least feasible allergen challenge model for regular use in pharmaceutical research.
EARLY ASTHMATIC RESPONSE ALLERGEN CHALLENGE MODEL
Several experimental asthma treatments have targeted inflammatory mediators involved in the EAR (e.g. IgE, histamine, leukotrienes) of which the EAR allergen challenge method was most appropriate.36-38 This model is similar to the classic allergen model in terms of the calculation for the starting allergen concentration and the use of 2-minute tidal breathing at 12-minute intervals. However, the final concentration administered is not necessarily the same for each challenge; doubling allergen concentrations are administered until the desired percent fall in FEV1 (typically 15% or 20%) is achieved; this allows for assessment of a dose response and quantitation of therapeutic benefit by way of dose shift in allergen PC15 or PC20. As a safety precaution for those who may develop a late response, inhaled corticosteroids (ICS) are routinely administered to attenuate the response once the maximal EAR has been captured or, if the recovery phase is important to the study design, administration of ICS can be extended ≤3 hours after the last allergen inhalation.39
Advantages to this model include its practicality (i.e. shorter study visits), its ease of repeatability, the greater availability of subjects (i.e. EAR-only and dual responders are both eligible), and its improved discrimination between drugs that have a large (>50–75%) inhibitory effect on the EAR.34,36 The main limitation of this method is that it prevents the examination of any aspect of the LAR.
POTENTIAL FUTURE DIRECTIONS FOR ALLERGEN CHALLENGE TESTING
As our knowledge expands, research techniques are repeatedly updated in order to continue to grow our understanding of physiological mechanisms. The allergen challenge model is no exception. For example, in pharmaceutical research, the classic allergen model typically excludes participants who do not develop an LAR, which represents approximately 50% (30–70%) of allergic asthmatics.12,19 Considering that the treatments tested with this methodology will also be used in EAR-only responders, it seems inappropriate to exclude them from important drug trials investigating efficacy. In addition, the LAR is dependent on the allergen and/or the dose used for testing.12,19 We recommend that the current methodology be updated to include the enrolment of both dual EAR/LAR responders and EAR-only responders to gain a better understanding of the allergic response and how to treat it. A potential method for doing so is the merging of the classic and EAR allergen models into one method with two study groups: early responders and dual responders.
Another shortcoming of classic standard methodology and assessing dual responders is the limitation for collecting sputum or measuring AHR during the time frame between the EAR and LAR, as an intervention performed has the potential to influence the development of the LAR. If we include early responders, we may be able to gain insight into allergen-induced increases in AHR since early responders show an increase at 3 hours post allergen challenge.40 At 24 hours post challenge, early responders have also shown increased AHR,41 as well as increased eosinophils and fractional exhaled nitric oxide,42 but without developing a classic LAR. By using a method that incorporates both the classic dual and EAR models (i.e. follow only to 3 hours), or by including EAR-only responders in the classic model (i.e. follow for 7–10 hours and include 24-hour assessments), it may be possible to obtain new insights into the mechanism(s) of allergic asthma.
An additional alteration to the classic allergen challenge could be a change in the definition of an LAR response. There are presently different definitions for the classification of a LAR, but one feature that remains relatively standard is that the FEV1 must fall by ≥15% from baseline during the LAR timeframe. While some studies require that such a percentage fall only happens once, others require that it occurs twice or three times, possibly even consecutively. It has been remarked that the LAR has an arbitrary cut-off point of 15%, which likely has little clinical significance and could be reduced.31 Some studies have shown that the inclusion of participants who fell between 10–15% during the LAR time frame strengthened the data set, especially considering that these participants showed similar results to those who had met the current LAR criteria.31,42 Future allergen challenge models could potentially reduce the cut-off point for defining the LAR to 10% without negatively influencing study outcomes.
Future studies should also aim to develop a practical set of tests and patient history questionnaires to phenotype asthmatics. At present, the application of research findings in clinics relies heavily on mean data of study samples that include several disease phenotypes. As a result, variances in treatment effects based on phenotype are not observed through current allergen testing methods,43 which could otherwise help to reduce the time and expense of finding the right therapeutic intervention for a given patient.
Further research must be conducted on repeated low-dose, repeated high-dose, and natural exposure allergen challenge models to improve their standardisation, feasibility, and repeatability; the repeated low-dose method must be modified to produce consistent airway inflammation; the repeated high-dose allergen model requires a safe protocol that is characterised for its effects on all aspects of the allergic response (i.e. inflammation, AHR, EAR, and LAR); and the natural exposure allergen model cannot (at least not thus far) be conducted in a regulated manner with most allergen types. Nonetheless, the exploration of these models for future use could prove beneficial for clinical trials of particular drug types.
CONCLUSION
Several allergen challenge techniques have been developed or considered, each with its benefits and shortcomings (Table 1). The classic model triggers an EAR, LAR, airway inflammation, and AHR, and requires the smallest number of study visits. However, it does not accurately simulate natural allergic exposure. The EAR model is more feasible, eases participant recruitment, and better discriminates drug effects. It does not, however, allow for the investigation of the LAR. The repeated low and high-dose allergen challenges more closely resemble environmental exposure but the former method produces inconsistent results and the latter raises safety concerns. The segmental method is unique in that it allows direct sampling of airway secretions, but it is invasive and requires special training. Lastly, the natural exposure allergen model most closely emulates the natural allergic situation, but is the least practical. Future studies could improve these methods to maximise their clinical relevance and better identify beneficial treatment options for different asthma phenotypes.








