Presenters: Claire Hopkins,1 Joaquim Mullol2
1. Guy’s and St Thomas’ Hospitals, London, UK
2. Hospital Clínic, Institut D’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), University of Barcelona, CIBER Respiratory Diseases, Barcelona, Spain
Disclosure: Prof Hopkins has served as an advisory board member for GlaxoSmithKline, Optinose, Sanofi Genzyme, and Smith & Nephew. Prof Mullol has received advisory boards consultancy fees, fees for lectures, and grants for research projects from AstraZeneca, Genentech, Glenmark Pharmaceuticals, GlaxoSmithKline, Menarini, MSD, Mitsubishi-Tanabe Pharma, Mylan-Meda, Novartis, Procter & Gamble, Sanofi Genzyme and Regeneron, UCB, and Uriach Group.
Acknowledgements: Writing assistance was provided by Dr Julia Duffey, Manifold Medical Communications Ltd, Matlock, UK.
Support: The publication of this article was sponsored by Sanofi Genzyme Europe B.V. Sanofi Genzyme and Regeneron are committed to providing resources to advance research in respiratory medicine in areas of unmet medical needs among patients with poorly controlled asthma and nasal polyps.
Citation: EMJ Respir. 2020;8[1]:57-63.
Meeting Summary
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a persistent, chronic inflammatory condition of the nasal passages and paranasal sinuses.1 Patients with CRSwNP experience a range of symptoms, including smell impairment, nasal obstruction, nasal congestion, headaches, and postnasal drip.2 The high symptom burden experienced by patients with CRSwNP negatively impacts their health-related quality of life (HRQoL).1-3 Most patients with CRSwNP have a type 2 pattern of inflammation characterised by elevated levels of IL-4, IL-5, and IL-13 and tissue infiltration by eosinophils, lymphocytes, basophils, and mast cells.4-6 First-line management options are limited and endoscopic sinus surgery is used when pharmacological interventions are unsuccessful; however, a considerable proportion of patients experience symptom recurrence following surgery.3,6-9 Dupilumab is a human monoclonal antibody that binds to the IL-4 receptor α subunit and inhibits signalling of IL-4 and IL-13, both of which act as key drivers of type 2 inflammation.10-13 Dupilumab has been approved in the USA and the European Union (EU) as an add-on maintenance treatment in adult patients with inadequately-controlled CRSwNP.14,15
Dupilumab Efficacy in Patients with Chronic Rhinosinusitis with Nasal Polyps by History of Prior Sinonasal Surgery: Pooled Results From the SINUS-24 and SINUS-52 Phase III Studies
Professor Claire Hopkins
Patients with serious CRSwNP or who experience disease relapse following pharmacological interventions may undergo endoscopic sinus surgery. However, symptom reappearance following surgery is common, occurring in 50−80% of patients within 3−5 years.3,6-9,16-18 Moreover, patients may not experience an improvement in smell following multiple surgeries. This study investigated dupilumab efficacy by quantifying prior sinonasal surgeries and the time since last surgery in patients with severe CRSwNP refractory to the standard of care. Pooled efficacy and safety datasets up to Week 24 from the Phase III SINUS-24 and SINUS-52 studies were used for the analysis. SINUS-24 and SINUS-52 evaluated dupilumab on a background of mometasone furoate nasal spray compared with mometasone furoate nasal spray alone (placebo).19 Participants enrolled in SINUS-24 were randomly assigned (1:1) to receive either subcutaneous dupilumab 300 mg (n=143) or placebo (n=133) every 2 weeks (q2w) for 24 weeks.19 Patients in SINUS-52 were randomly assigned (1:1:1) to dupilumab 300 mg q2w for 52 weeks (n=150), dupilumab q2w for 24 weeks and then every 4 weeks for the remaining 28 weeks (n=145), or placebo q2w for 52 weeks (n=153). Rescue treatment with either systemic corticosteroids and/or surgery was permitted for study participants.19 Patients were categorised according to the number of prior surgeries and time since last surgery. At baseline, the proportion of patients in these categories was well balanced between the study arms. Efficacy was measured using several scores:20 nasal polyp score (NPS), scale: 0−4 in each nostril, 0: no nasal polyps, 8: large nasal polyps causing complete obstruction of the inferior nasal cavity; nasal congestion (NC) score, scale: 0−3, 0: no symptoms, 3: severe symptoms that are hard to tolerate and cause interference with daily activities; Lund–Mackay (LMK) score, scale: 0−24 (0−12 for each nostril), 0: healthy, 2: total opacification; loss of smell symptom score, scale: 0–3, 0: no symptoms, 3: severe symptoms; University of Pennsylvania Smell Identification Test (UPSIT), scale: 0–40, <19: anosmia; the 22-item Sino-Nasal Outcome Test (SNOT-22), range: 0–110, item score: 0–5, 0: no problem, 5: problem as bad as it can be.
A total of 458 individuals were included in this study. Baseline sinus disease, measured by NPS (p=0.0047), NC score (p=0.0151), and LMK score (p<0.0001), was significantly worse for those patients who had not received surgery compared with individuals who had undergone surgery.
This was also true for olfactory dysfunction measured by the UPSIT and loss of smell scores (both p<0.0001). At baseline, patients who had surgery <3 years previously were younger (mean age: 46.4 years), had a higher LMK score (19.96), and lower mean bilateral endoscopic NPS (5.41; all p<0.0001) compared with participants in the ≥3 to <5 years, ≥5 to 10 years, and ≥10 year groups.
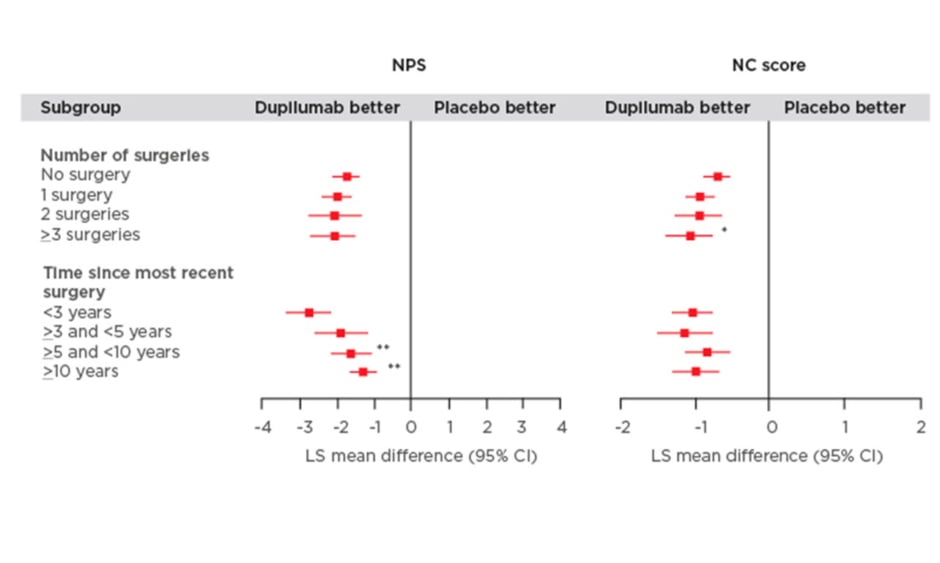
The study found that dupilumab improved all outcome measures irrespective of the number of prior surgeries or time since last surgery (Figure 1). Significantly greater improvements in the NPS were observed in patients who had a shorter time since last surgery (time since more recent surgery versus <3 years; p<0.05). Patients who received dupilumab with ≥3 surgeries had significantly higher NC scores compared with those individuals who did not have prior surgery (p<0.01; Figure 1). Dupilumab was also associated with greater improvements in both the LMK and UPSIT scores compared with placebo regardless of the time since last surgery or the number of prior surgeries (Figure 1). Significant improvements in LMK scores were seen in patients with a shorter time since last surgery compared to those with longer time since last surgery (<3 years versus >5 and <10 years; p<0.05, <3 years versus ≥10 years; p<0.001). Dupilumab also resulted in improvements in smell and SNOT-22 scores compared with placebo regardless of number of prior surgeries and time since last surgery.
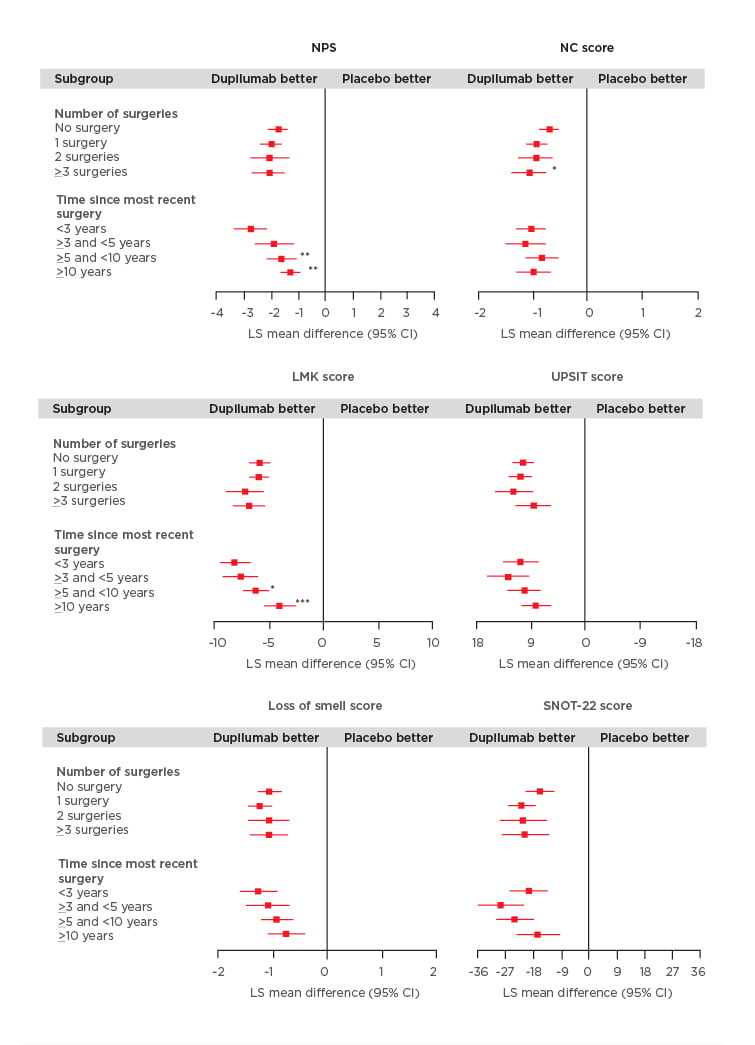
Figure 1: Efficacy outcomes using multiple measures assessing chronic rhinosinusitis with nasal polyp disease severity at Week 24 in patients receiving either dupilumab or placebo.
Subgroup-by-treatment interaction (comparing number of surgeries versus no surgery and time since more recent surgery versus <3 years).
*p<0.05
**p<0.01
***p<0.001
CI: confidence interval; LMK: Lund-Mackay; LS: least squares; NC: nasal congestion; NPS: nasal polyp score; SNOT-22: 22-item Sino-Nasal Outcome Test; UPSIT: University of Pennsylvania Smell Identification Test.
The efficacy of dupilumab was demonstrated over 24 weeks with a shorter time (<3 years) since last surgery associated with improved NPS and LMK scores, which may be indicative of high type 2 inflammation burden in these patients. Dupilumab reduced the need for rescue treatment with systemic corticosteroid and/or NP surgeries versus placebo, regardless of the number of prior surgeries or the time since last surgery within 10 years (Figure 2). Dupilumab had a favourable tolerability profile in individuals with and without prior surgery. The most common treatment emergent adverse events occurring in ≥5% of patients were nasopharyngitis (dupilumab 12.5% versus placebo 14.5%), nasal polyps (dupilumab 2.7% versus placebo 11.7%), and injection-site erythema (dupilumab 6.3% versus placebo 7.8%). This study demonstrated that over 24 weeks dupilumab consistently improved multiple measures of CRSwNP disease severity regardless of the number of prior sinonasal surgeries or time to last surgery.
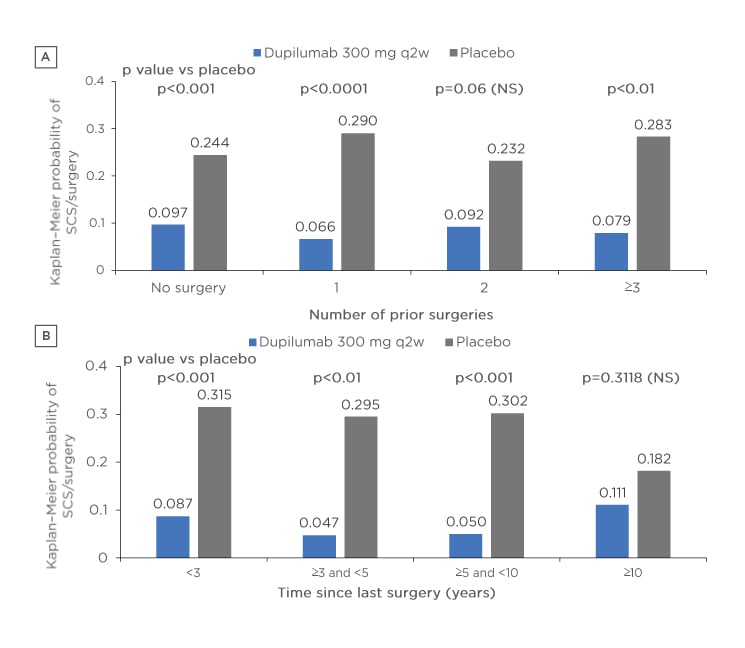
Figure 2: Probability of the need for rescue treatment and/or surgery according to A) number of prior surgeries and B) time since surgery in patients receiving either dupilumab or placebo.
p values derived from Cox proportional hazard models with the event of first systemic corticosteroid use and/or nasal polyp surgery (actual or planned, whichever is earlier) as the response variable, and study identifier, treatment, asthma/nonsteroidal anti-inflammatory drugs-exacerbated respiratory disease status, prior surgery history, and region (pooled countries) as covariates. No significant subgroup-by-treatment interaction.
NS: not significant; SCS: systemic corticosteroid; q2w: every 2 weeks.
Dupilumab Improved Smell Outcomes in Patients Irrespective of Years Since Chronic Rhinosinusitis with Nasal Polyps Diagnosis: Pooled Results From the SINUS-24 and SINUS-52 Phase III Studies
Professor Joaquim Mullol
Of all the symptoms experienced by patients with CRSwNP, the loss of smell is particularly troublesome and refractory to existing therapies.21,22 Loss of smell can be correlated with disease severity and has a considerable negative impact on an individual’s HRQoL.21-23
The objective of this study was to evaluate the effect of dupilumab on the individuals’ sense of smell in patients with severe CRSwNP using pooled data from SINUS-24 and SINUS-52 and to categorise these data according to the time since CRSwNP diagnosis. The study design of SINUS-24 and SINUS-52 was described in a previous review. Pooled data from SINUS-24 and SINUS-52 were analysed post hoc and the time since first diagnosis of CRSwNP was categorised into four groups: <5 years, 5 to <10 years, 10 to <15 years, and ≥15 years. Sense of smell outcomes at 24 weeks were determined by the loss of smell symptom score, UPSIT, and the smell/taste item of the SNOT-22 test.
Pooled data for each study arm and the time from diagnosis groups were analysed by the least square mean difference and 95% confidence interval (CI) of the change from baseline to Week 24. Furthermore, the odds ratio (95% CI) and risk difference (95% CI) were calculated for the absence of anosmia (UPSIT ≥19) for dupilumab versus placebo at Week 24. Findings from different subgroups were compared using the treatment-by-subgroup interaction p value.
A total of 719 patients were included in this analysis: <5 years, n=236; 5 to <10 years, n=157; 10 to <15 years, n=118; ≥15 years, n=208.
Most study participants were male (n=435; 60.5%) and at baseline there were significantly more patients in the ≥15 years subgroup who had ≥1 prior surgery (n=187; 89.9%; p<0.0001) compared with other time from diagnosis groups. Baseline data found that in severe, inadequately-controlled CRSwNP, a longer duration of CRSwNP was associated with greater impairment in the sense of smell.
This study found that in multiple measures of smell loss, dupilumab improved the sense of smell compared with placebo irrespective of the time from diagnosis (Figure 3). Significant differences were reported for all time from diagnosis groups (p<0.0001) except the <5 years subgroup. Furthermore, the investigation found that the proportion of patients in each subgroup without anosmia at Week 24 was significantly higher in all time from diagnosis groups for those receiving dupilumab than patients receiving placebo (nominal p<0.0001 derived using a linear probability model with treatment group, study, asthma/nonsteroidal anti-inflammatory drugs-exacerbated respiratory disease status, prior surgery history, and region as covariates). The study concluded that dupilumab improved smell outcomes from baseline to Week 24 in all patient subgroups regardless of the time from diagnosis. These results highlight the sense of smell improvements associated with dupilumab for patients with severe CRSwNP who otherwise have few therapeutic options and poor HRQoL.21-23
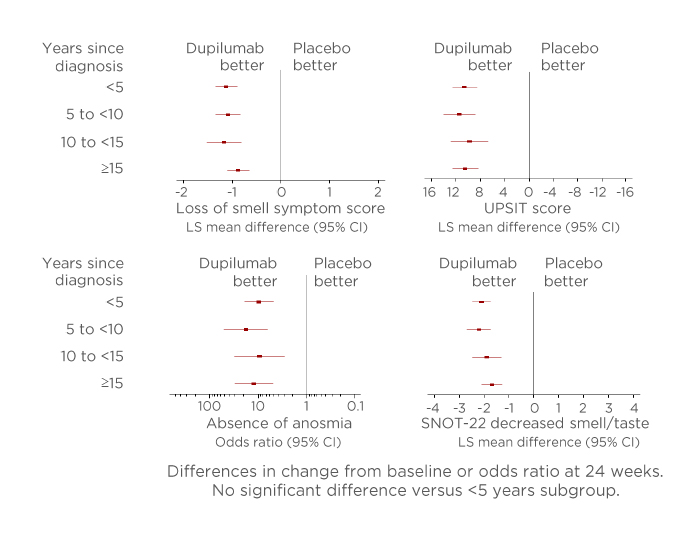
Figure 3: Outcomes in measures of sense of smell from baseline to Week 24 according to years since chronic rhinosinusitis with nasal polyp diagnosis.
Absence of anosmia: UPSIT ≥19. In each subgroup, each of the imputed complete data was analysed by fitting an ANCOVA model for quantitative parameters with the corresponding baseline value, treatment group, asthma/nonsteroidal anti-inflammatory drugs-exacerbated respiratory disease status, prior surgery history, and regions as covariates. The interaction p value was computed by fitting a similar ANCOVA model plus the time since diagnosis and the time since diagnosis-by-treatment interaction, using the <5 years subgroup as a reference. There was no significant difference versus the <5 years subgroup (p<0.05).
ANCOVA: analysis of covariance; CI: confidence interval; LS: least squares; SNOT-22: 22-item Sino-Nasal Outcomes Test; UPSIT: University of Pennsylvania Smell Identification Test.
MAT-EU-2000369 October 2020