Abstract
The estimated prevalence of urethral stricture disease is 229–627 per 100,000 males, though there are regional variations. Trauma, either from external force or iatrogenic causes, is currently the most common single cause of urethral stricture, although, as with prevalence, there are geographical variations. The presentation usually occurs with lower urinary tract symptoms, sometimes with urinary retention and, rarely, with watering can perineum. The symptoms are best evaluated with a combination of the American Urological Association (AUA) Symptom Index and urinary flow rate measurements for both new cases and suspected recurrences.
Time-tested retrograde urethrography remains the gold standard for a confirmatory diagnosis; however, it is limited by its inability to evaluate the posterior urethra and associated morbidities, such as abscesses and fistulas, thus three-dimensional imaging techniques are emerging as adjunct investigations. These modalities are not currently used universally, but their unavailability is not expected to be a serious hindrance to decision-making by a versatile reconstructive urologist.
Urethroplasty is regarded as the gold standard treatment for urethral stricture; excision and primary anastomosis, buccal mucosa graft, skin graft, and pedicle flap techniques have all been used. Notably, buccal mucosal graft urethroplasty has gained popularity above the others because of its versatility and success rate; this is considered to equate to urethral tissue engineering, which is at present confined to only a few centres.
INTRODUCTION
Urethral stricture disease is common and constitutes a large proportion of the urologist’s workload. The prevalence is estimated to be 229–627 per 100,000 males1 and its effects on the quality of life of those with the disease are far-reaching. In the UK, according to NHS statistics,2 since the start of the 21st century, 12,000 men have required an operation for urethral stricture, at an annual cost of £10 million and with an increasing prevalence in young men to 1 in 1,000 in men >65 years. Ekeke and Amusan3 documented a male-to-female ratio of 31.3:1.0 in Port-Harcourt, Nigeria, indicating that urethral stricture is very rare in females.
Gonococcal urethritis has been surpassed by strictures from trauma, lichen sclerosus (LS), transurethral resection of the prostate, open prostatectomy, post hypospadias repair, instrumentation, radiotherapy, and catheterisation.4,5 There are, however, temporal and geographical variations in the prevalence of the disease and the contribution of the different aetiologies.6 This, to a large extent, depends on the level of development and available healthcare resources.7 In the developing world, there are differences and variations in the reported contribution of the different aetiologies;7,8 however, there is uniformity in reporting trauma as the predominant aetiology in the developed world.9,10 Many authors have documented the bulbar urethra as being most commonly involved.8,9
The treatment of urethral stricture often depends on the expertise available; patients’ treatment choices have continued to evolve over the years.6 Dilatation was the first treatment used, but it has become disfavoured with many urologists now preferring urethroplasty, irrespective of the site and aetiology.11 In a nationwide survey in the Netherlands, direct vision internal urethrotomy was practiced by 97% of urologists, whereas urethroplasty was performed by only 6–23%.12 Urethrotomy is also falling out of favour as urethroplasty techniques continue to be refined. The aim of this review is to assess the current aetiology and evaluation of men with urethral stricture and the role of urethroplasty in the management of anterior urethral strictures.
AETIOLOGY OF URETHRAL STRICTURE
Historically, gonococcal urethritis was most commonly responsible for stricture formation in the urethra. Early studies1,4,9 indicated that urethritis was the most common cause, accounting for up to 50% in some series. Urethral stricture patients also presented late with complications such as urinary retention and watering can perineum; this posed serious challenges to treatment.13 Urethritis has now been surpassed in most parts of the world by trauma.
In the USA, >2.8 million people are hospitalised annually because of trauma, costing approximately $406 billion per year in medical expenses and productivity loss.14 Pelvic fracture results from high energy impact and is often indicated by the presence of urethral or bladder injury, or both. Pelvic fracture urethral distraction injury occurs in 4–19% of male pelvic fractures and 0–6% of female pelvic fractures and has been documented to have a prevalence of 5–25%;4 however, the National Trauma Data Bank® (NTDB) recently placed this figure at 1.4%.15 This narrowing of the posterior urethra is referred to as stenosis. Urethral injury with subsequent narrowing may also result from a fall astride or penetrating injury from a stab or gun shot.
Many authors have reported iatrogeny as the most common cause of urethral stricture, particularly in the developed world where healthcare intervention is more prevalent. Such interventions include hypospadias repair, cystoscopy, transurethral resection of the prostate, open prostatectomy, radiotherapy, and urethral catheterisation. Lumen et al.5 documented iatrogenic trauma from the above as the most common cause of urethral stricture, whereas only 19.07% in the study by Ekeke and Amusan3 were iatrogenic in origin. Urethral stricture has been documented to occur in 2% of men following external beam radiotherapy and 12% after brachytherapy.16 Malignancy of the urethra may actually masquerade as urethral stricture.
LS is considered an autoimmune inflammatory disease with a predilection for the anogenital region.17,18 According to Barbagli et al.,19 the true incidence of urethral involvement in patients with genital LS is unknown. However, approximately 8–16% of men affected by LS are said to develop urethral stricture and, while it is the most common cause of panurethral stricture,4,18 it accounts for 13.5% of the aetiology of urethral stricture.20 In some men, a cause for stricture cannot be found and these cases are therefore referred to as either congenital or idiopathic. According to Mundy,21 many idiopathic strictures are so called because their cause has been forgotten over the years; they are extremely common in the developed world.22
DIAGNOSIS OF URETHRAL STRICTURE
History and Physical Examination
Historically, male urethral stricture patients present with progressively increasing lower urinary tract symptoms with or without an obvious cause. According to Alwaal et al.,4 patients experience weak stream, straining to urinate, incomplete emptying, post-void dribbling, urinary retention, and recurrent urinary tract infection. Complications such as Fournier’s gangrene, obstructed ejaculation, urethrocutaneous fistulas, and acute or recurrent prostatitis and epididymoorchitis may be obvious at presentation. A weak stream, frequency, and incomplete voiding were, according to Nuss et al.,23 noted to be the most prevalent symptoms in patients undergoing urethroplasty for anterior urethral stricture. As the bladder decompensates, it becomes palpable, and acute or chronic urinary retention may occur. Overall, the symptoms are those of bladder outlet obstruction and, in cases that present late, perineal fistulas may be seen. A combination of urinary flow rate and the American Urological Association (AUA) Symptom Index is useful in early diagnosis of new cases and suspected recurrences.24
Imaging for Urethral Stricture
The confirmatory diagnostic procedure of choice for urethral stricture should be able to locate the stricture, determine the site, and assess the depth of spongiofibrosis.25 Currently available procedures do not individually combine these qualities; therefore, diagnosis requires direct visualisation, sonography, and contrast imaging. Retrograde urethrography (RUG) (Figure 1) is the gold standard in the investigation of urethral stricture disease.26 In the static type (Figure 1B and 1C), the film is taken following injection of the contrast. The posterior urethra is not visualised, as the contrast is milked into the bladder before the film is obtained. Therefore, this type cannot be completely relied on in posterior urethral disease as it visualises only the anterior urethra.27 The dynamic type (Figure 1A) is done under fluoroscopy for the immediate diagnosis of urethral disease.
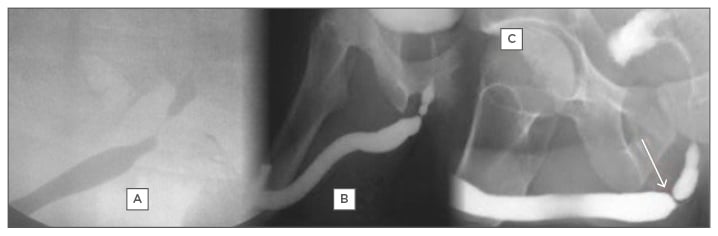
Figure 1: Retrograde urethrography images: A) dynamic; B) static; C) static showing bulbar stricture.
Dynamic RUG has the advantage of being able to visualise the posterior urethra and has a sensitivity and specificity of 90%.28 However, limitations include the requirement for clinical expertise, exposure to sepsis, anaphylactic reaction to contrast media, and associated radiation risk.
Voiding cystourethrography (VCUG) provides information on the dynamics of voiding and occult processes proximal to the stricture, but has the tendency to underestimate length in the bulbar urethra.20 Both VCUG and RUG can completely overlook complicating features such as fistulas, diverticula, and abscesses, because of which three-dimensional (3D) imaging modalities are emerging in the evaluation of urethral stricture disease.
Three-Dimensional Imaging
Ultrasonography (US) is an adjunct to RUG in defining the degree of spongiofibrosis and has better precision in diagnosing anterior urethral stricture. US has the capacity to delineate complications, such as abscesses and diverticula, and it is presently considered to be more precise in determining stricture length and location.21 US has comparable accuracy to RUG and magnetic resonance urethrography (MRU); it is, however, more successful than RUG in the characterisation of urethral lumen. Similar to the static RUG, US is unable to assess the posterior urethra.29
Magnetic resonance imaging (MRI) was first used in 1992 in an attempt to overcome the above limitations of RUG, VCUG, and US.30 It has been found to be able to determine the degree of urethral distraction, direction of prostate displacement, and defect length.31 Findings on MRU correlate more with intraoperative findings than RUG and VCUG, and it provides better information on spongiofibrosis.32 MRU limitations include cost, claustrophobia, need for expertise, limited availability, and incompatibility with implants.
Three-dimensional computerised tomographic urethrography is able to evaluate the location and length of distraction defect, the relationship of the pelvic bone to the urethra, and associated pathology in posterior urethral stricture.33 There are presently no indications that 3D imaging techniques, particularly MRI, are commonly used in the evaluation of men with urethral stricture.
The Role of Urethroplasty in the Treatment of Anterior Urethral Stricture
Urethral stricture disease remains the most common indication for urethroplasty. Over the decades, several urethroplasty techniques have emerged and continue to be refined as the pathology and dynamics of urethra stricture become clearer. Its use as a gold standard treatment of this disease22 is presently hampered by the limited number of trained reconstructive urologists and, according to Heyns et al.,34 limited theatre space, the presence of comorbidities, increased age, and, in some climes, speed of surgery and rural geography.35 According to the National Practice Survey Board of Certified Urologists in the United States,36 approximately half of American urologists do not perform urethroplasty, implying that this modality is not fully utilised. Data from the NHS in the UK during 2006 showed that direct vision internal urethrotomy or urethral dilatation was used in 93% of cases and urethroplasty in only 7%.37
Controversies also exist regarding the use of flaps and grafts, one-stage and staged procedures, and dorsal or ventral onlay, particularly in LS-induced and post hypospadias repair urethral strictures.38 On the whole, a good urethroplasty technique should be easily reproducible and result in a straight, good calibre urine stream. Direct vision internal urethrotomy is the most commonly performed procedure for urethral stricture despite its poor success rate. The current recommendation considered the most cost-effective is to refer patients for urethroplasty after a single failed urethrotomy.10
Bulbar Urethra
The bulbar urethra is the segment most amenable to different urethroplasty techniques. It is easily accessed (Figure 2A) for excision and primary anastomosis (EPA), one-stage substitution, and staged substitution urethroplasty with flaps or graft using buccal mucosa or skin.38
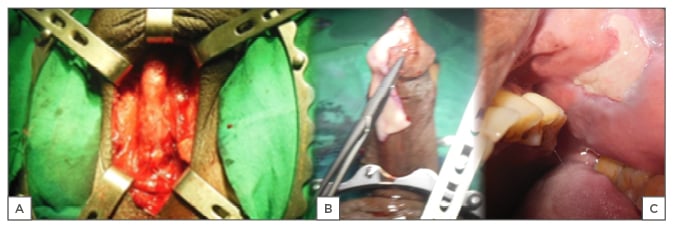
Figure 2: A) Exposed urethra; B) buccal mucosa; C) donor site.
Excision and Primary Anastomosis
Following exposure, as in Figure 2A, the urethra is dissected circumferentially, the extent depending on the length of the stricture, often extending to the bulb proximally and the penobulbar junction distally. Scar tissue is completely excised and the two ends of the urethra are spatulated and anastomosed around an indwelling catheter. Excessive excision of the urethra causes buckling of the penis and this may cause painful penile erection and uncomfortable sexual intercourse.39 The bulbar urethra can accommodate excision of 2 cm length, which is currently regarded as the comfort zone beyond which chordee may occur, though there are authors who advocate that up to 5 cm of this segment can be excised safely.40
Siegel and Murey41 expressed the opinion that EPA is the treatment of choice in men with bulbar urethral stricture following a 25-year meta-analysis of contemporary EPA. With a success rate of 90%, it was concluded that it is superior to other methods of treatment. Currently, urologists are concerned about transecting the urethra,42 particularly in post hypospadias repair, and as such EPA is falling out of favour. Zimmerman and Santucci11 referred to the ascendance of BMG urethroplasty above EPA as the preferred procedure for bulbar urethral stricture. However, it still has a strong indication in traumatic strictures, as the urethra is considered to have been transected at the time of the injury, especially in men with reasonable comorbidities.
Buccal Mucosa Graft Urethroplasty
BMG was introduced by Humby in 1941.43 It has favourable characteristics, such as early growth and graft survival, hairlessness, and compatibility with wetness, and has therefore emerged as a great reconstructive material for urologists (Figure 2B). Following exposure of the urethra (Figure 2A), a dorsal, ventral, or lateral stricturotomy approach may be used to patch the urethra.6 Kulkarni and Barbagli44 advocate placement of the graft ventrally in the proximal bulbar urethra and dorsally in the distal segment, depending on the clinical situation; this is also the author’s current practice. Ventral onlay requires less urethral dissection and has been documented as providing excellent results irrespective of the site and aetiology of the stricture.45 In BMG urethroplasty, the donor site may be closed or left open (Figure 2C); the latter has the advantage of causing less pain and numbness.
The BMG technique is particularly suited for men with LS-induced urethral stricture, in whom the use of skin is contraindicated, and in post hypospadias repair, in whom urethral transaction is of immense concern.46 The use of flaps in bulbar urethroplasty has waned as a result of the remarkable success achieved with BMG, which has been documented at >90%.47 Many renowned reconstructive urologists currently prefer bulbar stricturotomy and a BMG patch;6,48 however, flaps are still useful in redo cases when scarring is marked and the blood supply is less than adequate.49
Staged Urethroplasty
A staged approach to the reconstruction of the bulbar urethra is still sometimes adopted. Indication for this includes a hypoplastic urethral plate, the presence of infection, multiple fistulation either from the infection or trauma of urethral dilatation, previous failed repair, and the presence of comorbidities; in these cases, the urethra cannot be graft enlarged.50 The urethra can be exposed (Figure 2A) using a median raphe or a U-shaped incision followed by a ventral stricturotomy, which allows for inspection of the urethral plate. If the plate is judged to be unhealthy, it is marsupialised to the skin and retubularisation is completed in 3–6 months. In LS where the use of skin is contraindicated, the hypoplastic urethra is completely excised and replaced with BMG or patched dorsally during the first stage. It has been documented that perineal urethrotomy is well accepted in communities accustomed to seated or squatted voiding.51
PENDULOUS URETHRA
BMG, skin graft, flaps, one-stage and staged urethroplasty are all applicable in penile urethra reconstruction, unlike EPA, which is contraindicated because resection of even a short segment of the penile urethra causes chordee of the penis.52 Barbagli et al.53 recently described their approach to reconstruction of the penile urethra in men with stricture of this segment. They advised that in patients with LS, the use of buccal mucosa at the first stage is mandatory because LS does not affect the oral mucosa, while those with a history of failed hypospadias repair presenting with obliterative strictures, associated fistulas, scarred penile skin, chordee, abnormal meatus, small glans, and deficiency of the dartos layer may require a two-stage repair, using the buccal mucosa in the second stage. According to them, the majority of patients presenting with penile strictures not related to LS or failed hypospadias repair are good candidates for one-stage urethroplasty using a graft or flap.
In the one-stage technique, the urethra is opened ventrally, patched dorsally with a BMG, and closed ventrally (Asopa).54 In the staged procedure, the dorsal BMG patch is done during the first stage, but the urethra is sutured to the adjacent skin margin and retubularisation is done in the second stage. However, in the Johansson staged urethroplasty, the dorsal BMG patch is omitted while the urethra is marsupialised to the skin (Figure 3A). The urethra is expanded by taking adjacent skin with it during retubularisation at the second stage. This approach has its advocates, disadvantages, advantages, indications, and contraindications.52,55,56
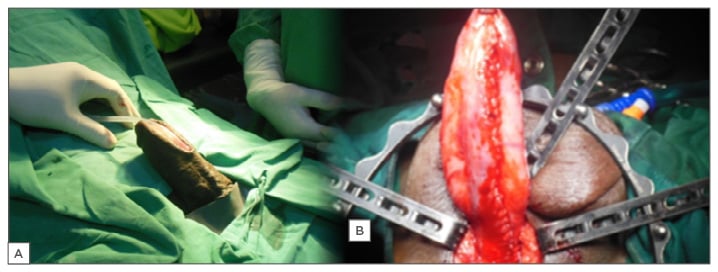
Figure 3: A) Second-stage Johansson’s urethroplasty; B) Kulkarni’s panurethroplasty.
Orandi55 and Quartey56 described the reconstruction of the anterior urethra using a pedicled skin flap. The principles of these single-stage procedures still remain valid in the treatment of non-obliterative penile urethral strictures that are not LS-induced. The multiplicity of these techniques requires the approach to each patient to be individualised, taking into consideration the patient’s desire, aetiology of the stricture, previous failed repairs, and the sexual functioning of the penis.
PANURETHRAL STRICTURE
Panurethral stricture poses a great challenge to the reconstructive urologist, because of the high level of surgical experience and expertise it requires, and also to the patient, as repair has a significant potential for morbidity. Two distinct approaches are described here.
Kulkarni’s Panurethroplasty
Kulkarni et al.57 described a one-stage reconstruction of the entire urethra in men with panurethral stricture. This involves exposing the urethra via a perineal incision (Figure 2). Through the incision, a one-sided dissection and penile invagination allows the entire anterior urethra to be exposed. This is followed by a dorsal stricturotomy and a BMG patch (Figure 3B). It requires harvesting of buccal mucosa bilaterally and it is therefore an extensive surgery.
Kulkarni et al.57 documented a success rate of 84.9% with this procedure and described the advantages as including the perineal approach, which avoids a penile incision and suture line, minimisation of the risk of urethrocutaneous fistula, excellent cosmesis, reduced incidence of chordee, and preservation of the bulbospongiosus muscle.
Staged Approach
Panurethral reconstruction can also be approached in two stages by repairing the bulbar segment using a ventral or dorsal on-lay BMG urethroplasty and first-stage Johansson (Figure 3A) for the penile urethra at the same setting. The penile urethra is tubularised by augmenting it with adjacent penile skin or dorsal BMG in the second stage. This was described by Zimmerman and Santucci11 in 2011, but appears to have been overshadowed by the panurethroplasty of Kulkarni, because staged procedures are said to be fraught with technical issues such as multiple revisions.58
Technical expertise, complexity of the stricture, the state of the urethral plate, presence of comorbidities and infection will sometimes favour the use of a staged approach. A single-stage panurethroplasty is an enormous undertaking and therefore requires effective patient selection, as a perineal urethrotomy may even be acceptable and adequate for some men with reasonable comorbidities. The authors have seen men with a stone lodged in their urethra proximal to the stricture with severe infection and poorly controlled diabetes.
Tissue Engineering
Substitution urethroplasty using skin or buccal mucosa is the standard treatment for urethral stricture disease. Substitution surgery is associated with increased morbidity and limited availability of substitute material. This led to the development of interest in urethral tissue engineering. Results of tissue engineered repairs are currently considered to be similar to standard repairs, though its use at present is limited to failed previous repairs in a few centres.59