Author: Christos Evangelou1
1. MedRight Medical Writing & Editing, San Diego, California, USA
Disclosure: Evangelou has declared no conflicts of interest.
Disclaimer: Trastuzumab deruxtecan has not been approved by the FDA for the neoadjuvant or adjuvant setting for early-stage, HER2-positive breast cancer. Trastuzumab deruxtecan is currently approved for the treatment of adult patients with unresectable or metastatic HER2-positive breast cancer who have received a prior anti-HER2-based regimen either in the metastatic setting, or in the neoadjuvant or adjuvant setting, and have developed disease recurrence during or within 6 months of completing therapy; and for the treatment of adult patients with unresectable or metastatic hormone receptor-positive, HER2-low, or HER2-ultralow breast cancer that has progressed on one or more endocrine therapies or chemotherapy in the metastatic setting.
Support: The publication of this article was funded by AstraZeneca.
Keywords: Adjuvant therapy, antibody–drug conjugates (ADC), HER2+ early breast cancer, neoadjuvant therapy, pathologic complete response (pCR).
Citation: Oncol AMJ. 2025;2[Suppl 4]:2-12. https://doi.org/10.33590/oncolamj/KXTT3305
Meeting Summary
Data presented at the European Society for Medical Oncology (ESMO) Congress 2025 signal a potential shift in the management of early-stage human epidermal growth factor receptor 2 (HER2)-positive (HER2+) breast cancer. Results from the pivotal Phase III DESTINY-Breast05 and DESTINY-Breast11 trials suggest that trastuzumab deruxtecan (T-DXd) improves outcomes in curative-intent settings. The DESTINY-Breast05 trial demonstrated a statistically significant improvement in invasive disease-free survival (IDFS) with T-DXd compared to trastuzumab emtansine (T-DM1) in the adjuvant setting for patients with high-risk residual disease, reducing the risk of recurrence or death by 53%. This benefit was consistent across subgroups, and the safety profile of T-DXd was manageable. In DESTINY-Breast11, treatment with T-DXd followed by an anthracycline-free neoadjuvant regimen (paclitaxel, trastuzumab, and pertuzumab) resulted in a pathologic complete response (pCR) rate of 67.3%, with a more favorable safety profile than that of traditional anthracycline-containing therapy. These findings suggest a potential shift in treatment approaches for high-risk early-stage HER2+ breast cancer, with incorporation of antibody–drug conjugates (ADC) in pre- and post-operative settings to improve efficacy and tolerability compared to conventional chemotherapy backbones.Introduction
HER2+ breast cancer accounts for 15–20% of all breast cancer cases and is characterized by the overexpression or amplification of the HER2 gene.1,2 HER2+ breast cancers exhibit an aggressive tumor biology and have historically had a poor prognosis, including a high risk of recurrence.3,4 However, the introduction of HER2-targeted therapies, beginning with trastuzumab, has improved outcomes for patients with HER2+ disease, providing disease-free survival (DFS) comparable to that of patients with HER2-negative breast cancer.5
The current therapeutic strategy for high-risk early-stage HER2+ breast cancer aims to maximize the pathologic response to neoadjuvant systemic therapy, which typically involves a combination of anthracycline-based chemotherapy and dual HER2 blockade (trastuzumab and pertuzumab).6 Although pCR is a strong surrogate marker for overall survival (OS) and local-regional control in patients with breast cancer undergoing neoadjuvant chemotherapy, up to 50% of patients with HER2+ breast cancer show residual invasive disease (RID) after neoadjuvant chemotherapy and trastuzumab-based treatment.7,8 Patients with RID, particularly those with hormone receptor-negative disease, have an elevated risk of distant recurrence and death.8 In addition, current neoadjuvant chemotherapy regimens can cause acute hematological and gastrointestinal toxicity,9-11 as well as cardiotoxicity and secondary leukemia.12,13 The low rates of pCR and high toxicity of current neoadjuvant treatments underscore the need for effective and safe perioperative treatments for high-risk patients with HER2+ breast cancer.
T-DM1 is the current standard of care in the adjuvant setting for patients with early-stage HER2+ breast cancer who have RID, as established by the Phase III KATHERINE trial.14,15 The study showed that adjuvant treatment with T-DM1 after completion of neoadjuvant therapy with a trastuzumab-containing regimen significantly improved IDFS compared with adjuvant trastuzumab alone.14 However, long-term IDFS survival benefits with T-DM1 among patients with advanced locoregional disease or positive nodal status after neoadjuvant therapy are limited, with 7-year IDFS rates of 67% and 72%, respectively.16 Moreover, adjuvant treatment with T-DM1 did not reduce the rate of central nervous system (CNS) recurrence compared with trastuzumab.16 These data emphasize the need for more effective treatments for high-risk patients in the post-neoadjuvant setting.
This review provides an up-to-date synthesis of the latest clinical data from recent Phase III trials in early-stage HER2+ breast cancer presented at the ESMO Congress 2025, focusing on data marking a potential paradigm shift for ADCs moving into perioperative curative-intent treatment of early-stage disease (Table 1). This review of recent clinical data aims to support informed clinical decision-making among clinicians treating patients with early-stage HER2+ breast cancer.
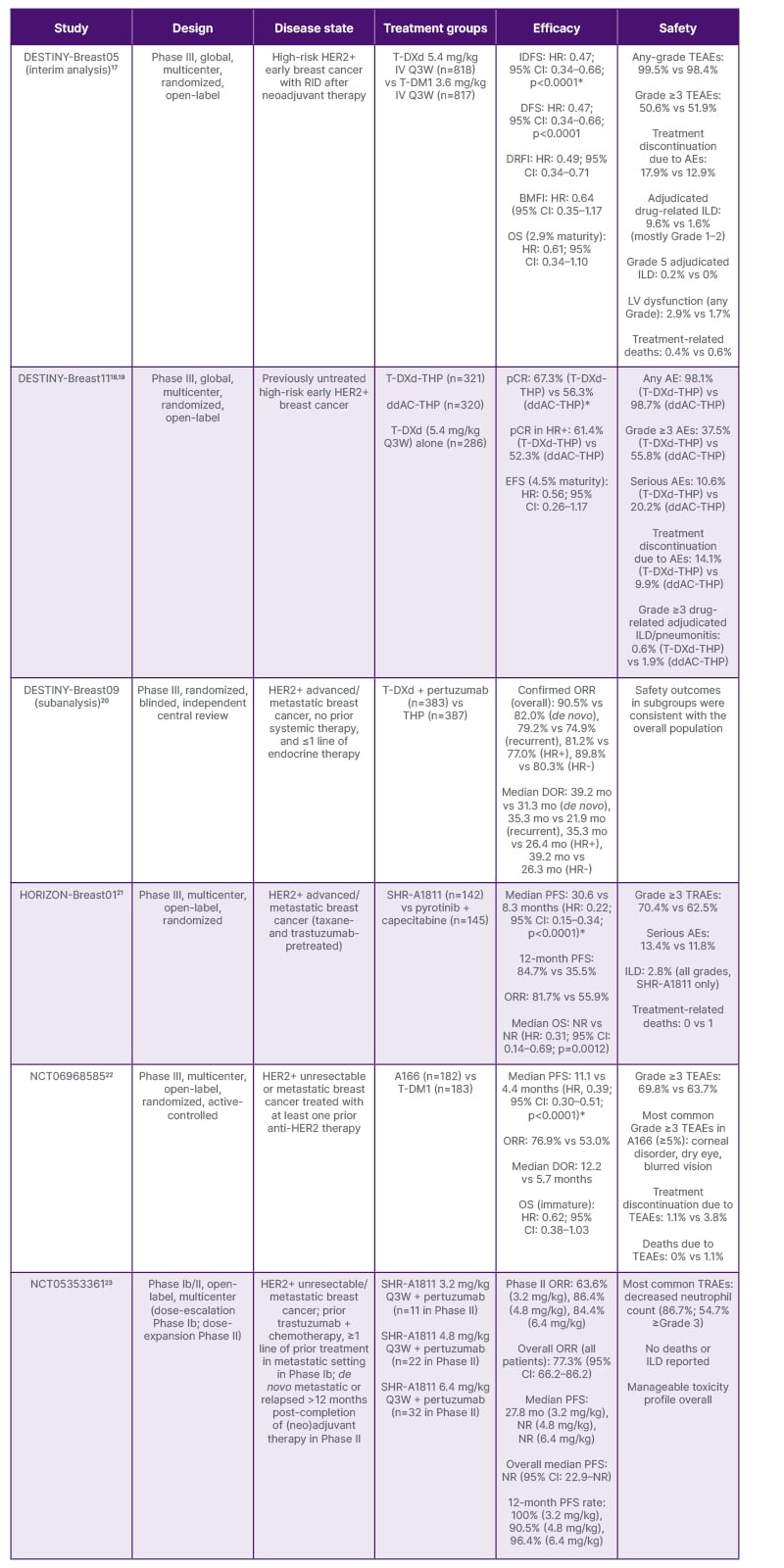
Table 1: Summary of recent data from antibody–drug conjugates trials in HER2+ breast cancer presented at the ESMO 2025.
*Primary endpoint.
A166: trastuzumab botidotin; ADC: antibody–drug conjugate; AE: adverse event; BMFI: brain metastasis-free interval; ddAC-THP: dose-dense doxorubicin and cyclophosphamide followed by THP; DOR: duration of response; DRFI: distant recurrence-free interval; EFS: event-free survival; HER2: human epidermal growth factor receptor 2; HR: hazard ratio; HR+: hormone receptor-positive; HR–: hormone receptor-negative; IDFS: invasive disease-free survival; ILD: interstitial lung disease; NR: not reached; LV: left ventricular; mo: months; ORR: objective response rate; OS: overall survival; pCR: pathologic complete response; PFS: progression-free survival; Q3W: every three weeks; RID: residual invasive disease; T-DM1: trastuzumab emtansine; T-DXd: trastuzumab deruxtecan; TEAE: treatment-emergent adverse event; THP: taxane + trastuzumab + pertuzumab; TRAE: treatment-related adverse event; vs: versus.
Advances in Adjuvant Therapy for Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Interim Analysis of DESTINY-Breast05
Charles E. Geyer Jr., breast medical oncologist and professor of medicine at the University of Pittsburgh UPMC Hillman Cancer Center, Pennsylvania, USA, started his talk at the ESMO Congress 2025 by highlighting the unmet clinical needs in patients with HER2+ breast cancer who show residual disease after neoadjuvant treatment, including the low long-term IDFS rates and the relatively high rates of CNS recurrence in patients treated with T-DM1 in the KATHERINE trial.16 He also noted that the clinical rationale for investigating T-DXd in the high-risk post-neoadjuvant setting is supported by the activity of T-DXd in early phase studies in heavily pretreated patients with HER2+ or HER2-low metastatic breast cancer and the superiority of T-DXd relative to T-DM1 in the second-line metastatic setting in the DESTINY-Breast03 trial.24,25
Study Design and Patient Population
DESTINY-Breast05 is a global, open-label, randomized Phase III trial that enrolled 1,635 patients across 481 sites.17 Eligible patients had centrally confirmed HER2+ disease (immunohistochemistry 3+ or in situ hybridization-positive) with RID in the breast and/or axillary lymph nodes following at least 16 weeks of neoadjuvant chemotherapy that included at least 9 weeks of taxane-based therapy and 9 weeks of trastuzumab with or without pertuzumab.17
The trial focused on high-risk patients, defined as either inoperable early breast cancer (cT4, N0–3, M0 or cT1–3, N2–3, M0) before neoadjuvant therapy, or operable disease (cT1–3, N0–1, M0) with axillary node-positive disease (ypN1-3) after neoadjuvant treatment.17 Patients were randomized 1:1 to receive either T-DXd 5.4 mg/kg or T-DM1 3.6 mg/kg intravenously every 3 weeks for 14 cycles. The primary endpoint was IDFS.
In his presentation, Geyer emphasized that over half of the patients presented with T4 or N2–3 disease, and 80% had positive nodal status after neoadjuvant therapy,17 which is higher than the 46% seen in KATHERINE.16 Nearly 80% of patients received dual HER2-targeted neoadjuvant treatment, and 93% received adjuvant radiotherapy, with 54–59% administered concurrently with the study treatment.17
Efficacy Results
At the interim analysis (data cutoff: July 2nd, 2025), with a median study duration of 30 months and 153 IDFS events (74% information fraction), T-DXd demonstrated a 53% reduction in the risk of invasive disease recurrence or death compared with T-DM1.17 The 3-year IDFS rate was 92.4% (95% CI: 89.7–94.4%) with T-DXd and 83.7% (95% CI: 80.2–86.7%) with T-DM1 (hazard ratio [HR]: 0.47; 95% CI: 0.34–0.66; p<0.0001), representing an absolute difference of 8.7%.17
The IDFS benefit with T-DXd was consistent across all prespecified subgroups, including age, race, geographic region, hormone receptor status, disease presentation status before neoadjuvant therapy, post-neoadjuvant pathologic nodal status, type of HER2-targeted neoadjuvant therapy, and timing of radiotherapy.17
DFS, the key secondary endpoint of the study, was improved with T-DXd compared with T-DM1, with 3-year DFS rates of 92.3% and 83.5%, respectively (HR: 0.47; 95% CI: 0.34–0.66; p<0.0001). OS analysis at 2.9% maturity showed 18 deaths with T-DXd versus 29 with T-DM1, and 3-year OS rates of 97.4% and 95.7%, respectively (HR: 0.61; 95% CI: 0.34–1.10).17
Patterns of Recurrence
The distant recurrence-free interval showed an HR of 0.49 (95% CI: 0.34–0.71), with a 3-year distant recurrence-free interval rate of 93.9% versus 86.1%.17 Although brain metastasis-free interval data remain immature, a clinically meaningful trend favoring T-DXd was observed (HR: 0.64; 95% CI: 0.35–1.17), with 3-year brain metastasis-free interval rates of 97.6% versus 95.8%.17
Analysis of first IDFS events demonstrated that the occurrences of local invasive recurrence, regional recurrence, and contralateral disease were all lower with T-DXd than with T-DM1.17 Distant recurrences occurred in 42 patients receiving T-DXd, compared with 77 patients receiving T-DM1, with CNS involvement in 17 and 25 patients, respectively.17
Safety Profile and Tolerability
Treatment completion rates were high in both arms, with 72.3% of patients in the T-DXd arm and 76.3% of those in the T-DM1 arm completing all 14 planned cycles.17 Median treatment duration was approximately 10 months in both groups. Grade 3 or higher treatment-emergent adverse events (TEAE) occurred in 50.6% of patients in the T-DXd arm and 51.9% of those in the T-DM1 arm.17
The most common any-grade TEAEs with T-DXd were nausea (71.3%), constipation (32.0%), decreased neutrophil count (31.6%), and vomiting (31.0%).17 Although gastrointestinal toxicities such as constipation, vomiting, and diarrhea were relatively frequent with T-DXd, they were predominantly Grade 1 or 2. Radiation pneumonitis occurred at similar rates in both arms (28.8% with T-DXd versus 27.0% with T-DM1), and was predominantly Grade 1.17
During the presentation, Geyer explained that interstitial lung disease (ILD) is a significant safety concern with T-DXd. Adjudicated drug-related ILD occurred in 9.6% of T-DXd-treated patients compared with 1.6% of T-DM1-treated patients.17 In both treatment groups, most ILD cases were Grade 1 or Grade 2, with seven (0.9%) Grade 3 events and two (0.2%) Grade 5 (fatal) events in the T-DXd group. The timing of radiotherapy (sequential versus concurrent) did not affect the incidence of ILD in any of the treatment groups.17 Geyer emphasized that most ILD cases were reversible with appropriate monitoring and timely intervention, and that detailed data on ILD reversibility from the DESTINY-Breast05 trial will be presented at the San Antonio Breast Cancer Symposium® (SABCS®).
Left ventricular dysfunction occurred at low rates in both arms (2.9% with T-DXd and 1.7% with T-DM1), with predominantly Grade 2 events (2.5% with T-DXd and 1.4% with T-DM1).17 TEAEs led to death in three (0.4%) T-DXd-treated patients (two from ILD/pneumonitis, one from respiratory tract infection adjudicated as not ILD) and five (0.6%) T-DM1-treated patients (from various causes).17
Clinical Implications
Geyer emphasized that adjuvant T-DXd represents a potential new standard of care in the management of high-risk HER2-positive breast cancer with residual disease after neoadjuvant therapy, noting that the 53% reduction in the risk of invasive disease recurrence or death is substantial and clinically meaningful, particularly given the high-risk nature of the disease in this population.17 He added that the consistency of benefit across subgroups and the reduction in both systemic and locoregional recurrences, including a numerical reduction in CNS metastases, suggest a benefit across a broad patient population. Geyer also noted that, although the rates of adjudicated drug-related ILD were under 10%, with the majority being Grade 1 or 2 and reversible, appropriate monitoring remains essential.17
Advances in Neoadjuvant Therapy for Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Insights From DESTINY-Breast11
In her presentation at ESMO, Nadia Harbeck, medical oncologist and professor of conservative oncology at LMU University Hospital, Munich, Germany, emphasized the unmet need for more effective and less toxic neoadjuvant regimens for early-stage HER2+ breast cancer.19 She explained that current standard neoadjuvant regimens combining trastuzumab and pertuzumab with polychemotherapy result in pCR benefit in high-risk patients and high rates of acute and long-term adverse events. She also noted that the improved survival outcomes with T-DXd compared to T-DM1 or taxane in combination with trastuzumab and pertuzumab in patients with advanced or metastatic HER2+ breast cancer26,27 supported the rationale for testing T-DXd in the neoadjuvant setting in the DESTINY-Breast11 trial, the findings of which were simultaneously published.18
Study Design and Patient Population
The Phase III, open-label DESTINY-Breast11 trial enrolled 927 patients across 147 sites in 18 countries, randomizing them 1:1:1 to three arms: T-DXd alone for eight cycles, T-DXd for four cycles followed by paclitaxel plus trastuzumab and pertuzumab (T-DXd-THP) for four cycles, or dose-dense doxorubicin and cyclophosphamide followed by THP (ddAC-THP) as the control arm.18,19 The study enrolled patients with high-risk features, requiring a tumor stage of ≥cT3 and N0–3, or a primary tumor of any size with positive lymph node involvement.18,19 Approximately 1% of enrolled patients had inflammatory breast cancer.19
The median age of participants was 50 years, and nearly half of the patients were from Asia.19 Harbeck emphasized the high-risk features of the study cohort, with approximately 73% presenting with hormone receptor-positive disease, 89% with node-positive disease, and 50% with clinical Stage III disease.18,19 The T-DXd monotherapy arm closed early following an Independent Data Monitoring Committee recommendation in March 2024, based on a lower pCR rate and low likelihood of superiority versus the control. This decision was not driven by safety concerns.18
Trastuzumab Deruxtecan Followed by Paclitaxel Plus Trastuzumab and Pertuzumab Improves Pathologic Complete Response Rates
The trial met its primary endpoint of pCR.18,19 Treatment with T-DXd-THP resulted in a pCR rate of 67.3%, compared to 56.3% with ddAC-THP, representing an absolute improvement of 11.2 percentage points (95% CI: 4.0–18.3; p=0.003).19 Harbeck noted that this difference crossed the prespecified significance threshold and represents a clinically meaningful improvement.
Among patients with hormone receptor-positive disease, who typically have low response rates, pCR rates were 61.4% with T-DXd-THP and 52.3% with ddAC-THP.19 In patients with hormone receptor-negative disease, pCR rates were 83.1% and 67.1%, respectively. pCR benefits were consistent in most prespecified subgroups, including age groups, menopausal status, and disease stage.19 The improvement was observed regardless of whether patients had immunohistochemistry 3+ or other HER2 status.19
Residual cancer burden analysis confirmed the improvements in pCR with T-DXd-THP. After surgery, 81.3% of patients receiving T-DXd-THP had no or minimal residual invasive cancer (RCB-0 or RCB-I) compared to 69.1% with ddAC-THP.19 In the hormone receptor-positive subgroup, 78% achieved RCB-0+I with T-DXd-THP, versus 65% with ddAC-THP.19
Early Survival Signals
Although still immature (4.5% event maturity), event-free survival data suggest a favorable trend for T-DXd-THP, with an HR of 0.56 (95% CI: 0.26–1.17) compared to ddAC-THP.19 At 24 months, 96.9% of patients in the T-DXd-THP arm remained event-free, compared with 93.1% in the control arm.19 Post-neoadjuvant treatments were well balanced between arms, with more than half of patients without pCR receiving T-DM1 as recommended by the study protocol.18,19 Final event-free survival analysis is planned when all patients reach 3 years of follow-up.18
Safety Profile and Tolerability
T-DXd-THP demonstrated improved toxicity compared to anthracycline-based therapy. Grade 3 or higher adverse events occurred in 37.5% of patients receiving T-DXd-THP versus 55.8% with ddAC-THP.19 Serious adverse events were reported in 10.6% and 20.2% of patients, respectively.19
The incidence of adjudicated drug-related ILD was low in all treatment arms (4.4% with T-DXd-THP, 4.9% with T-DXd alone, and 5.1% with ddAC-THP).18 Grade ≥3 ILD was less common with T-DXd-THP (0.6%) than with ddAC-THP (1.9%).19
Hematological and cardiac toxicities were lower with T-DXd-THP than with ddAC-THP. Neutropenia of any grade occurred in 29.1% with T-DXd-THP compared to 44.2% with ddAC-THP, and anemia rates were 22.8% and 49.7%, respectively.19 Left ventricular dysfunction occurred in 1.3% of patients in the T-DXd-THP arm (including one Grade ≥3 event), compared to 6.1% of those in the ddAC-THP arm (including six Grade ≥3 events).19 No cardiac failure events occurred with T-DXd-THP or T-DXd.18 However, peripheral neuropathy was more common with T-DXd-THP (25.9%) than with ddAC-THP (20.8%).19
Clinical Implications
In her presentation, Harbeck emphasized that DESTINY-Breast11 opens the door to anthracycline-free, ADC-anchored neoadjuvant strategies for high-risk HER2+ breast cancer. She noted that DESTINY-Breast11 results support T-DXd-THP as a more effective and less toxic neoadjuvant treatment compared with ddAC-THP, adding that T-DXd-THP may become a preferred regimen for patients with high-risk, early-stage HER2+ breast cancer.19
The multimodal mechanism of T-DXd, which combines HER2-targeted delivery with a potent topoisomerase I inhibitor payload and a bystander effect, appears to translate effectively from the metastatic to the early disease setting.18 The sequential approach of T-DXd followed by THP may optimize both efficacy and tolerability by introducing the ADC early while maintaining dual HER2 blockade throughout treatment.18
Other New-Generation Human Epidermal Growth Factor Receptor 2-Targeted Antibody–Drug Conjugates for Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer
Although T-DXd was the only ADC discussed at the ESMO Congress 2025 that showed a shift into the curative setting, the Congress featured emerging data on other new-generation ADCs being tested in patients with HER2+ in the advanced or metastatic setting.
SHR-A1811
The HORIZON-Breast01 trial investigated SHR-A1811, a HER2-directed ADC that has previously demonstrated single-agent antitumor activity in heavily pretreated solid tumors.21 The multicenter Phase III study enrolled 287 patients with advanced or metastatic HER2+ breast cancer previously treated with taxanes and trastuzumab.21 Patients were randomized to receive either SHR-A1811 or the combination of pyrotinib (a pan-HER tyrosine kinase inhibitor) plus capecitabine, which represents standard second-line care in China.21 The median progression-free survival (PFS) was 30.6 months with SHR-A1811, compared with 8.3 months with the comparator regimen (HR: 0.22; 95% CI: 0.15–0.34; p<0.0001).21 Although median OS data are immature, the authors reported early signs of OS advantage with SHR-A1811 (HR: 0.31; 95% CI: 0.14–0.69; p=0.0012).21 The objective response rate (ORR) also favored SHR-A1811 at 81.7% versus 55.9%. The incidence of treatment-related adverse events was comparable between arms, with Grade 3 or higher treatment-related adverse events occurring in 70.4% of patients treated with SHR-A1811.21 ILD occurred in 2.8% of patients receiving SHR-A1811, and most ILD cases were low grade.21
Trastuzumab Botidotin
ESMO 2025 featured data from a Phase III trial comparing trastuzumab botidotin (A166), another HER2-directed ADC, with T-DM1 in patients with metastatic HER2+ breast cancer.22 The study enrolled 365 patients who had received at least one prior anti-HER2 therapy, with over half having been exposed to two or more lines and 55.9% having received prior pyrotinib. A166 provided a median PFS of 11.1 months, compared with 4.4 months with T-DM1 (HR: 0.39; 95% CI: 0.30–0.51; p<0.0001). The PFS benefit was consistent across subgroups regardless of prior treatment lines.22 The ORR favored A166 at 76.9% versus 53.0%, and the median duration of response was 12.2 months and 5.7 months, respectively. Although OS data are immature, early data suggest a trend favoring A166 (HR: 0.62; 95% CI: 0.38–1.03).22 The most common (≥5%) Grade ≥3 TEAEs were ocular toxicities (corneal disorder, dry eye, and blurred vision).22 Most ocular events with A166 were manageable, with functional limitations resolving in the majority of affected patients. Treatment discontinuation rates were low in both arms (1.1% for A166, 3.8% for T-DM1).22
SHR-A1811
According to data presented at the Congress, the combination of SHR-A1811, a novel HER2-targeted ADC combining trastuzumab linked to a topoisomerase I inhibitor payload, with pertuzumab showed early signs of antitumor activity in patients with unresectable or metastatic HER2+ breast cancer who had previously received trastuzumab-based therapy.23 In the Phase II portion of this study, 65 patients were treated with one of three doses of SHR-A1811 (3.2, 4.8, and 6.4 mg/kg), administered every 3 weeks in combination with pertuzumab. The ORR was 63.6%, 86.4%, and 84.4% with the 3.2 mg/kg, 4.8 mg/kg, and 6.4 mg/kg doses, respectively.23 The median PFS was not reached at a median follow-up of 22 months, and the 12-month PFS rates were 100.0%, 90.5%, and 96.4%, respectively. The safety profile was manageable, with neutropenia being the most frequent treatment-related adverse event, occurring in 86.7% of patients (with 54.7% Grade 3 or higher). No cases of ILD were reported.23
Conclusions
The recent clinical data presented at the ESMO Congress 2025 point to a potential paradigm shift in the management of early-stage HER2+ breast cancer, with ADCs moving from the metastatic setting into curative-intent perioperative treatment. DESTINY-Breast05 establishes T-DXd as a potential new standard of care in the adjuvant setting for patients with residual disease after neoadjuvant therapy, demonstrating a clinically meaningful 53% reduction in recurrence risk compared to T-DM1.17 This improvement is clinically meaningful, particularly given the consistent benefit observed across patient subgroups and the reduction in both distant and locoregional recurrences in high-risk patients.
DESTINY-Breast11 provides evidence that anthracycline-free neoadjuvant regimens anchored by T-DXd can achieve superior pCR rates with a more favorable toxicity profile compared with conventional chemotherapy.18,19 These findings open the door to eliminating anthracyclines in select high-risk patients, potentially reducing long-term cardiac and hematologic sequelae while improving pCR rates.
Although T-DXd demonstrated a generally manageable safety profile, the integration of T-DXd into perioperative care will require vigilance for early detection and management of ILD.17–19 This should include patient education, baseline pulmonary assessment, and serial low-dose chest CT monitoring.28-31 Careful patient selection will also be critical, particularly in patients with pre-existing lung disease or concurrent radiotherapy.28-31







