Interview Summary
Globally, there are more than 650,000 newly diagnosed cases of bladder cancer annually, with up to approximately 80% of these cases being non-muscle invasive bladder cancer (NMIBC). Approximately 25% of NMIBC cases are classified as high risk. The current standard treatment for high-risk NMIBC is intravesical instillation of Bacillus Calmette-Guérin (BCG). Up to 40% of patients with high-risk NMIBC have disease recurrence or progression on this treatment. The standard of care for BCG-unresponsive high-risk NMIBC has traditionally been radical cystectomy; however, this is a complex, life-altering procedure that is associated with substantial morbidity, considerable impact on quality of life, and a 90-day post-surgery mortality rate of up to 8%. Many patients are ineligible for radical cystectomy or refuse this surgery. There is an unmet medical need for effective, bladder-sparing treatments for patients with BCG-unresponsive high-risk NMIBC who wish to preserve their bladder or are too frail for major surgery. For this article, EMJ conducted interviews in September 2025 with three key opinion leaders: Ashish Kamat from the Department of Urology, University of Texas, MD Anderson Cancer Center, Houston, USA; Joshua Meeks from the Department of Urology, Northwestern Medicine, Chicago, Illinois, USA; and Félix Guerrero-Ramos from the Department of Urology, Hospital Universitario 12 de Octubre, Madrid, Spain, to discuss recent developments in BCG-unresponsive NMIBC research. The experts discussed the diagnosis of patients with NMIBC with carcinoma in situ (CIS) with or without papillary disease, and current treatment approaches for BCG-unresponsive high-risk NMIBC. In addition, they looked at the complete response (CR) and duration of response (DOR) results in patients with BCG-unresponsive, high-risk NMIBC with CIS with or without papillary disease (Cohort 2) in the SunRISe-1 study. They also highlighted the importance of considering both CR and DOR to give a complete picture of overall clinical benefit. Following this, Kamat, Meeks, and Guerrero-Ramos described the safety profile of gemcitabine intravesical releasing system (Gem-iDRS) and quality-of-life data in Cohort 2 of the SunRISe-1 study. The next topic of discussion was the disease-free survival (DFS) rates in BCG-unresponsive high-risk papillary disease-only NMIBC (Cohort 4) in the SunRISe-1 study. Finally, the experts outlined the changing landscape and potential future developments in BCG-unresponsive NMIBC clinical practice and research in the context of the recent approval of the Gemcitabine Intravesical System (Gem-iDRS), as well as advances in diagnosis, treatment, and patient support they would like to see.
INTRODUCTION
There are over 650,000 newly diagnosed cases of bladder cancer per year globally.1 Approximately 75–80% of these cases are NMIBC,2,3 of which approximately 25% are classified as high risk.4 Up to 40% of patients with high-risk NMIBC have disease recurrence or progression during or after intravesical BCG therapy, which is the current standard treatment for high- and some intermediate-risk patients.3,5,6 The standard of care for BCG-unresponsive high-risk NMIBC has traditionally been radical cystectomy;6 however, this complex, life-altering procedure is associated with substantial morbidity (surgical site infection, urinary tract infection, sepsis/septic shock, renal failure, venous thromboembolism),7 considerable impact on quality of life, and a 90-day post-surgery mortality rate of up to 8%.8,9 Furthermore, many patients are ineligible for radical cystectomy or are unwilling to undergo this surgery.3,10 There is an unmet medical need for effective, bladder-sparing treatments for patients with BCG-unresponsive high-risk NMIBC who would like to preserve their bladder or are too frail for major surgery.3
DIAGNOSIS OF PATIENTS WITH NON-MUSCLE INVASIVE BLADDER CANCER WITH CARCINOMA IN SITU WITH OR WITHOUT PAPILLARY DISEASE
CIS with or without papillary disease is found in at least 10% of BCG-unresponsive NMIBC cases and is underdiagnosed.11 Bladder CIS is a high-grade disease with a high risk of progression to the muscle layer that requires aggressive treatment.12 Kamat explained that the detection and diagnosis of CIS is crucial and is dependent on the careful evaluation of the patient by the urologist. Flat lesions such as CIS are often missed under standard white light cystoscopy.12 Blue light or fluorescent cystoscopy is more effective than regular white light for detecting tumours;12 however, blue light technology is not widely available.13
Meeks remarked that CIS can have a red appearance, but this does not necessarily help with the detection of initial disease or recurrence, as BCG-related inflammation and redness complicate the clinical picture. Meeks stated that “from a therapeutic perspective, the major challenge is to eradicate something you cannot see that is covering almost the entire bladder lining. It is beneficial to use blue light technology and biopsy areas that look normal to check for the presence of CIS.”
Guerrero-Ramos indicated that the first step in diagnosing bladder CIS is to encourage urologists to form partnerships with pathologists, to look for CIS using enhanced imaging methods, and to perform randomised biopsies in certain patients with positive cytology, noting that “if you don’t look for CIS, you will never find it.” It is also important for the urologist to inform pathologists about any suspicion of CIS to highlight a possible CIS diagnosis versus a dysplasia diagnosis. However, the collaboration between urologists and pathologists likely varies in different healthcare settings. Guerrero-Ramos indicated that rates of CIS detection tend to be higher in university hospitals than in community hospitals because the former are more likely to have access to the appropriate tools to aid detection of CIS. Guerrero-Ramos suggested that the variation in the rates of CIS diagnosis in high-grade, high-risk patients with NMIBC reported in the literature is likely due to the level of specialisation of the healthcare professionals involved and the extent of the collaboration within the multidisciplinary team.
CURRENT TREATMENT APPROACHES FOR BACILLUS CALMETTE-GUÉRIN-UNRESPONSIVE, HIGH-RISK, NON-MUSCLE INVASIVE BLADDER CANCER
Three treatments were approved in the USA between 2020–2024 for BCG-unresponsive high-risk NMIBC with CIS with or without papillary tumours in patients who either refuse or are ineligible for radical cystectomy: pembrolizumab,14,15 nadofaragene firadenovec,16,17 and nogapendekin alfa inbakicept plus BCG.18,19
Gem-iDRS is a novel intravesical drug-releasing system designed to provide prolonged delivery of gemcitabine for patients with BCG-unresponsive high-risk NMIBC.20,21 This intravesical drug-releasing system is administered once every 3 weeks through to Month 6, then once every 12 weeks through to Month 24.20,21
On 9th September 2025, the FDA approved Gemcitabine Intravesical System (Gem-iDRS) monotherapy for patients with BCG-unresponsive NMIBC with CIS with or without papillary tumours (only),22 based on the results from Cohort 2 of the Phase IIb SunRISe-1 study.23,24 This approval follows the FDA Breakthrough Therapy Designation and Real-Time Oncology Review programme as it provides another novel therapeutic option for patients who have previously had limited treatment choices.
Guerrero-Ramos, who has placed Gem-iDRS in more than 100 patients in clinical studies through a total of over 1,000 procedures (insertions and removals) since 2017, explained that “there has been a lot of clinical study activity in this area recently, which is great for our patients, and FDA approval of Gemcitabine Intravesical System (Gem-iDRS) monotherapy is an important milestone.”
In addition to these available approved therapies, gemcitabine–docetaxel sequential chemotherapy is also used in this patient population.25 Meeks specified that there has been no prospective data generated, and that the data on this treatment are based on the assembled experience of multiple cohorts.26
According to Meeks, the downside of gemcitabine–docetaxel sequential chemotherapy is the long duration of treatment, with patients given six doses followed by monthly maintenance. Each time, the patients are required to be in the hospital for at least 3 hours. However, there is an ongoing prospective study looking at this.
Guerrero-Ramos emphasised that enrolling patients in clinical trials is a viable and important management option to give patients access to newer therapies. If patients refuse to be enrolled in a clinical trial or are ineligible, or there is no suitable clinical trial available, physicians are encouraged to offer the most appropriate treatment available in their healthcare setting.
COMPLETE RESPONSE RATE AND DURATION OF RESPONSE IN BACILLUS CALMETTE-GUÉRIN-UNRESPONSIVE, HIGH-RISK NON-MUSCLE INVASIVE BLADDER CANCER WITH CARCINOMA IN SITU WITH OR WITHOUT PAPILLARY DISEASE (COHORT 2) IN THE SUNRISE-1 STUDY
The design of the SunRISe-1 study is shown in Figure 1.23,27
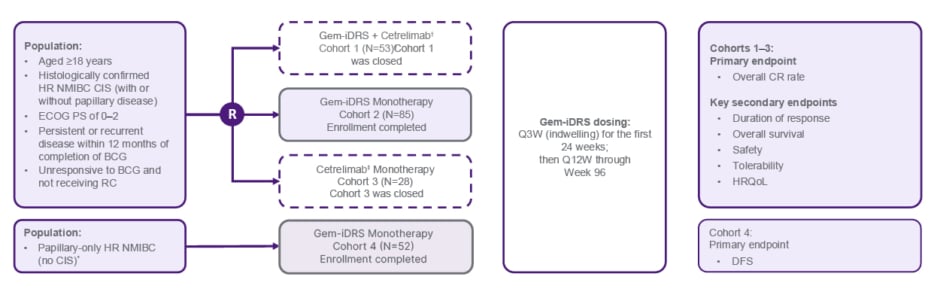
Figure 1: Phase IIb SunRISe-1 study: cohort 2 Bacillus Calmette-Guérin-unresponsive high-risk non-muscular invasive bladder cancer with carcinoma in situ with or without papillary disease.23,27
*Patients with BCG-unresponsive papillary-only HR NMIBC (high-grade Ta, any T1) per protocol amendment 4.
†Cetrelimab is an anti–programmed cell death-1; cetrelimab dosing was Q3W through Week 78.
‡Number of patients enrolled in Cohort 1 was N=55 and number of patients treated was N=53.
BCG: Bacillus Calmette-Guérin; CIS: carcinoma in situ; CR: complete response; DFS: disease-free survival; ECOG PS: Eastern Cooperative Oncology Group performance status; HR NMIBC: high-risk non-muscle invasive bladder cancer; HRQoL: health-related quality of life; Q3W: every 3 weeks; Q12W: every 12 weeks; R: randomisation; RC: radical cystectomy.
In the SunRISe-1 study Cohort 2 (patients with BCG-unresponsive NMIBC with CIS with or without papillary disease), 70/85 patients achieved CR. A total of 95.7% of responses (67/70 patients) were achieved at the first disease evaluation at 3 months (Figure 2), with a median (range) time to response of 2.8 (2.1–8.3) months.23,24
After a median (range) follow-up in responders of 20.2 (5–48) months, median DOR was 25.8 months (95% CI: 8.3–not estimable), with 52.9% of the responses (37/70 responses) lasting ≥12 months (Figure 2).25,26
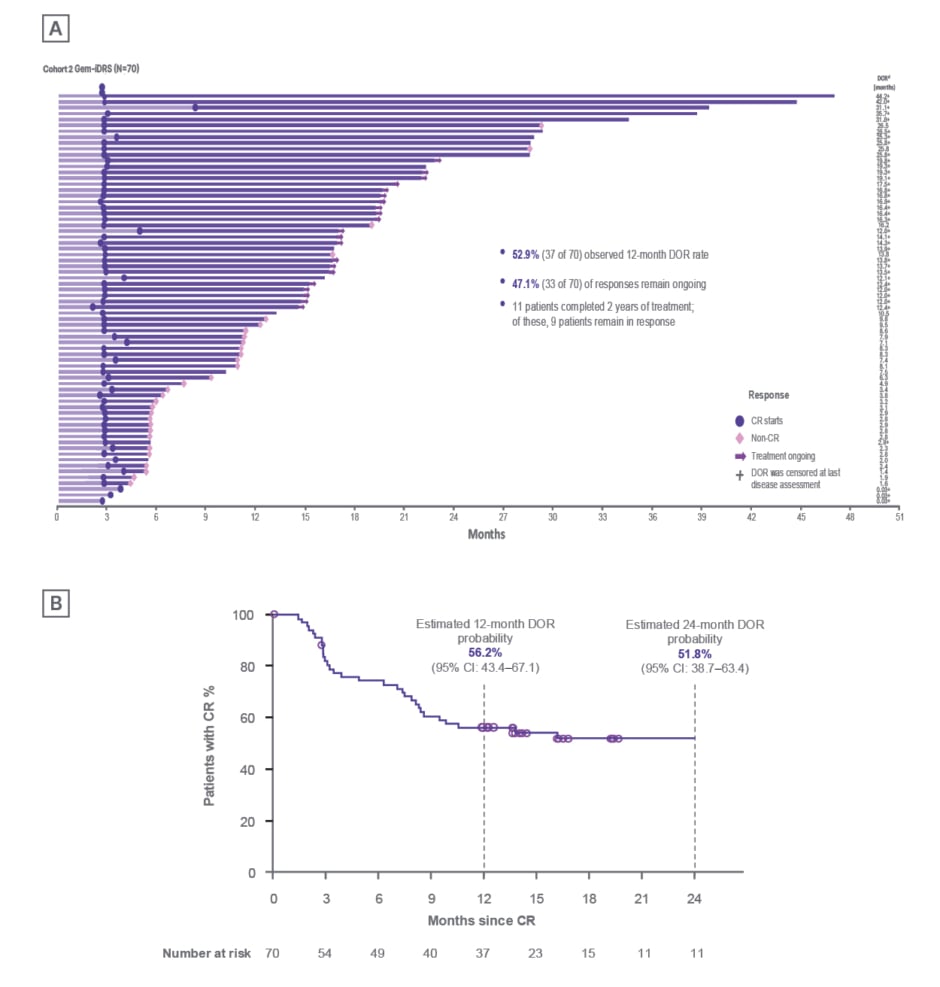
Figure 2: Durability of response to Gem-iDRS monotherapy in patients with carcinoma in situ with or without papillary disease (Cohort 2) in the SunRISe-1 study.24
A) Time to CR or non-CR in all patients (N=85), with DOR in individual responders (N=70). B) DOR (Kaplan–Meier curve). Timepoints with <10 patients at risk are excluded from the plot.
CR: complete response; DOR: duration of response.
Guerrero-Ramos commented that the results from Cohort 2 of the SunRISe-1 study confirm the results obtained from preliminary clinical trials conducted previously, including TAR-200-101,20 TAR-200-102,28 and TAR-200-103.29
Kamat summarised: “An ideal treatment is one that is not only effective, well tolerated, and safe, but also has a less intensive administration schedule in terms of the regularity of healthcare visits required. Based on the results of SunRISe-1, the Gem-iDRS appears to meet these criteria and is a critical step in the right direction for NMIBC treatment.”
Meeks explained that the short time to first response (median: 2.8 months) is essential from a healthcare provider’s perspective in terms of getting patients into remission: “Achieving a response really matters to patients, and a response early in treatment may encourage patients to be more positive and compliant with treatment.”
Kamat, Meeks, and Guerrero-Ramos specified that both CR and DOR endpoints should be considered together to provide a comprehensive picture of overall clinical benefit, rather than that provided by either endpoint alone, and to guide treatment decisions. According to the experts, while their patients clearly value treatment responses and bladder preservation, they regard durability of response as particularly important. Meeks emphasised that “patients want to know what their chances of keeping their bladders and being cancer-free in a year’s time are.”
Guerrero-Ramos emphasised: “The CR in the SunRISe-1 study is a CR after a first induction, as those patients who were not in CR were not allowed to undergo reinduction. The long duration of response is relevant because not only do we want a CR, we also want the response to be durable.” Guerrero-Ramos gave an example of considering CR and DOR together to drive treatment decisions. He described that patients on BCG who achieve a complete response but recur early (e.g., at 3 months) are recommended to undergo radical cystectomy.6 In contrast, patients with a complete and durable response on BCG, as well as late recurrence (e.g., at 4 years), may show clinically relevant responses to rechallenge with BCG on disease recurrence,30 thus indicating that BCG rechallenge could be an option for certain patients who recur following BCG treatment, and circumventing the need for bladder removal at this point. Guerrero-Ramos concluded that these treatment decisions are best made following consideration of both CR and DOR to optimise treatmentfor patients.
SAFETY PROFILE OF GEM-iDRS IN BACILLUS CALMETTE-GUÉRIN-UNRESPONSIVE, HIGH-RISK NON-MUSCLE INVASIVE BLADDER CANCER WITH CARCINOMA IN SITU WITH OR WITHOUT PAPILLARY DISEASE (COHORT 2) IN THE SUNRISE-1 STUDY
Kamat, Meeks, and Guerrero-Ramos emphasised that urologists are familiar with the types of treatment-related adverse events (TRAE) reported with Gem-iDRS monotherapy in patients with BCG-unresponsive high-risk NMIBC with CIS with or without papillary disease (Cohort 2) in the SunRISe-1 study (Table 1).24
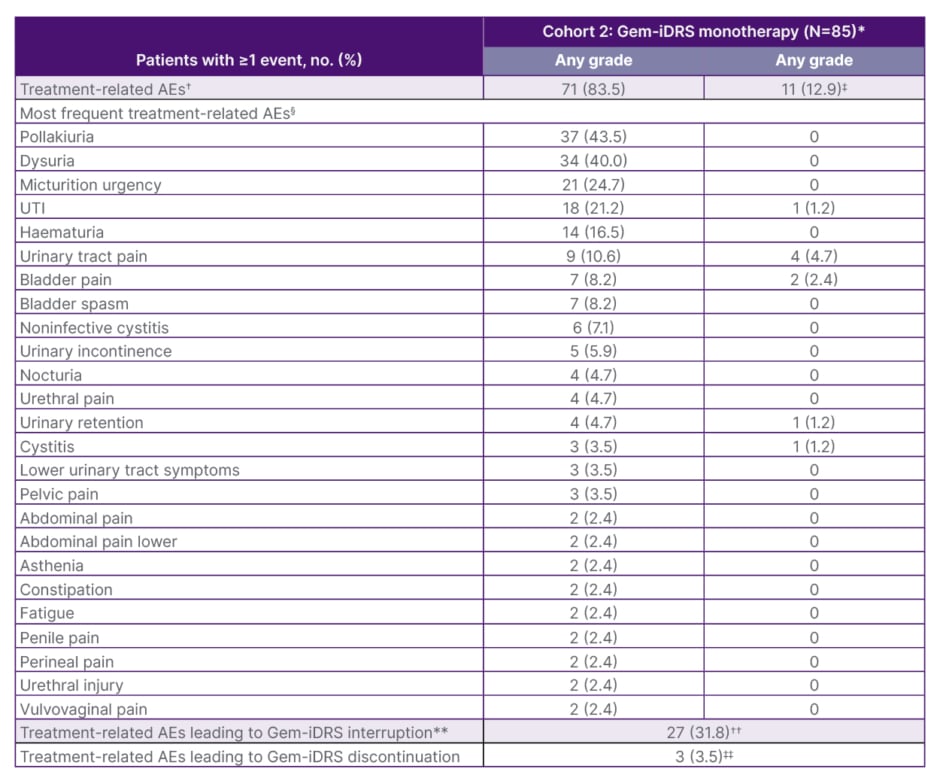
Table 1: Treatment-related adverse events, Gem-iDRS interruption, and discontinuation rates in patients with Bacillus Calmette-Guérin-unresponsive high-risk non-muscular invasive bladder cancer with carcinoma
in situ with or without papillary disease (Cohort 2) in the SunRISe-1 study.24
*Safety data are shown for all patients who received at least one dose of study drug in the full analysis set of the Gem-iDRS monotherapy in CIS with or without papillary disease cohort (N=85).
†An AE was categorised as related if the investigator determined there was a possible, probable, or causal relationship between the AE and the study drug/procedure. Patients were counted only once for any given event, regardless of the number of times they actually experienced the event.
‡In addition to the Grade ≥3 treatment-related AEs shown by preferred term in the table, all other Grade ≥3 percentage treatment-related AEs were reported in only one patient each and included acute kidney injury, pseudomonal cystitis, and urosepsis. Patients may have had one or more Grade ≥3 treatment-related AEs.
§Treatment-related AEs of any grade by preferred term are listed if they were reported in ≥2% of patients in the Gem-iDRS monotherapy in CIS with or without papillary disease cohort.
**Gem-iDRS interruption is defined as when a Gem-iDRS dose is skipped or Gem-iDRS is removed early.
††Most patients had one-to-two skipped Gem-iDRS doses, and common reasons for interruption included urinary tract pain (5.9%), pollakiuria (4.7%), and UTI (4.7%).
‡‡Treatment-related AEs leading to Gem-iDRS discontinuation included two patients (2.4%) with noninfective cystitis and one (1.2%) with pollakiuria and with urinary tract disorder. Patients who discontinued may have had one or more treatment-related AE.
AE: adverse event; CIS: carcinoma in situ; No: number; UTI: urinary tract infection.
A total of 71 (83.5%) TRAEs were reported in Cohort 2, with the most common being pollakiuria (37 [43.5%]), dysuria (34 [40.0%]), micturition urgency (21 [24.7%]), and urinary tract infection (18 [21.2%]).23,24 Most of the treatment-emergent adverse events reported in the study were Grade 1 or 2, and these events resolved after a median of 3.1 weeks (range: 0.11–150.31).23,24 Three patients (3.5%) discontinued Gem-iDRS because of TRAEs, including non-infective cystitis (n=2), and pollakiuria and urinary tract disorder (n=1).23,24 Kamat noted that the adverse events reported in the cohort are not only familiar to urologists, who are well equipped to manage these side effects, but also to the patients, which may have contributed to the low discontinuation rates.
Guerrero-Ramos noted that the TRAEs were usually lower urinary tract symptoms, which are common symptoms associated with routinely used intravescical therapies. The patients in this cohort had previously undergone several urological procedures and therapies, and they were likely familiar with symptoms such as haematuria (blood in the urine) and infections. Guerrero-Ramos commented that patients in clinical trials may try to minimise the number of adverse events that they report and the impact that these events have on their health and wellbeing because they are already familiar with the symptoms and know that they are not life-threatening; they want to continue with the treatment and do not want to be withdrawn from the trial.
Eleven patients (12.9%) had Grade ≥3 TRAEs.23,24 Five patients (5.9%) had at least one serious TRAE, with cystitis with bladder pain (Grade 2), pseudomonal cystitis (Grade 3), urinary tract infection (Grade 3), urosepsis with acute kidney injury (Grade 3), and urinary tract pain (Grade 3) reported in one patient each.23,24 There were no treatment-related deaths.23,24
The treatment interruption rate in Cohort 2 was 31.8% (27 patients),23,24 although Guerrero-Ramos expected this rate to be lower in future studies as investigators become more familiar with the Gem-iDRS and learn how best to manage side effects without interrupting treatment.
Similarly, Meeks considered that, although urologists are familiar with the TRAEs reported in Cohort 2, management of these events will likely continue to improve with time, experience, and specific training and support on Gem-iDRS placement for physicians and nurse practitioners.
QUALITY OF LIFE IN PATIENTS WITH BACILLUS CALMETTE-GUÉRIN-UNRESPONSIVE, HIGH-RISK NON-MUSCLE INVASIVE BLADDER CANCER WITH CARCINOMA IN SITU WITH OR WITHOUT PAPILLARY DISEASE (COHORT 2) IN THE SUNRISE-1 STUDY
There was no deterioration in the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – Core Questionnaire (EORTC QLQ-C30) Global Health Status and Physical Functioning quality-of-life measures in patients with BCG-unresponsive high-risk NMIBC with CIS with or without papillary disease (Cohort 2) in the SunRISe-1 Study (Figure 3).23
Mean Global Health Status and Physical Functioning scores were high at baseline (75.0 and 86.2, respectively) and stable on treatment (i.e., they did not exceed the clinically meaningful change threshold of 10 points),31-33 with these measures maintained at baseline levels up to the 100-day safety follow-up.23,24 The experts described the patient-reported quality-of-life outcomes in the SunRISe-1 study as encouraging.
Kamat commented that the cadence of treatments with Gem-iDRS in terms of healthcare visits, namely every 3 weeks for the first 6 months, then once every 3 months, likely contributes to the stability of the quality-of-life measures in the SunRISe-1 study.23
Meeks expressed: “To be able to provide therapy to patients that is not going to consume much nursing time or office resources is a novelty, and it is beneficial for patients to be able to go home in between visits while continuously receiving prolonged therapy with the intravesical drug releasing system.”
Guerrero-Ramos commented that patients on Gem-iDRS often keep working as well as participating in their daily activities, such as playing golf or tennis, and going swimming. Patients who like to travel are mindful of the time of insertion or removal of the iDRS.
DISEASE-FREE SURVIVAL RATES IN BACILLUS CALMETTE-GUÉRIN-UNRESPONSIVE HIGH-RISK PAPILLARY DISEASE-ONLY NON-MUSCLE INVASIVE BLADDER CANCER (COHORT 4)IN THE SUNRISE-1 STUDY
In patients with BCG-unresponsive high-risk papillary disease-only NMIBC (Cohort 4), the DFS rates with Gem-iDRS therapy at 6, 9, and 12 months were 85.3% (95% CI: 71.6–92.7), 81.1% (95% CI: 66.7–89.7), and 70.2% (95% CI: 51.6–82.8), respectively.24,27 Kamat explained that the DFS rates in Cohort 4 indicate that 14.7%, 18.9%, and 29.8% of patients had disease recurrence at 6, 9, and 12 months of treatment, respectively. Kamat commented that these data are encouraging and the highest reported DFS rates to date in this patient population; they show that Gem-iDRS therapy has activity in patients with BCG-unresponsive papillary-only disease. Please note this is not in the current label for Gem-iDRS.
Meeks explained that papillary-only NMIBC is usually associated with lower progression than NMIBC with CIS, and the emphasis of treatment is to prevent recurrence rather than to shrink the tumours.
Guerrero-Ramos pointed out that although CIS (with or without papillary disease) is a more challenging disease to treat than papillary-only disease because it is more aggressive, patients with papillary-only disease account for most cases (90%) of BCG-unresponsive NMIBC.11 To date, Guerrero-Ramos noted, all trials in patients with NMIBC with CIS with or without papillary disease have initiated an exploratory cohort for patients with papillary-only disease; however, the FDA will not approve any treatments for the papillary-only population based on single-arm trials. RCTs are therefore needed and ongoing in this population.
CONCLUSIONS AND FUTURE PROSPECTS
According to Kamat, “the outlook is increasingly promising for patients with BCG-unresponsive NMIBC. We now have several approved therapies, each with distinct efficacy and safety profiles, allowing us to individualise treatment strategies. The SunRISe-1 study has demonstrated that Gem-iDRS is both feasible and well tolerated, introducing a new therapeutic paradigm in this space.”
Kamat added that expanding therapeutic options for BCG-unresponsive disease remains essential, as most patients wish to pursue bladder-preserving strategies before considering cystectomy. “At the same time,” Kamat cautioned, “we must recognise when the priority should shift from bladder preservation to preventing progression and safeguarding survival so as to not miss the window of opportunity for cure. This is an area that continues to represent a major unmet need.”
Meeks stated that “approximately four in five patients with BCG-unresponsive high-risk NMIBC with CIS with or without papillary disease in Cohort 2 of the SunRISe-1 study had a CR at 3 months, with almost half of responders maintaining CR at 12 months. These clinical study data are meaningful for physicians and patients and are going to change how we treat our patients.” Meeks remarked that SunRISe-1 was a well-conducted study that has set a benchmark for other potential therapies in the management of BCG-unresponsive NMIBC.
Meeks predicted that the landscape of therapy for BCG-unresponsive NMIBC will change drastically over the next 12–24 months following the recent approval of the novel agents in this setting, which may result in better outcomes for patients.
Meeks is also looking forward with interest to the results of studies in other patient populations, concluding: “It is an incredibly exciting time to take care of patients with BCG-unresponsive NMIBC.”
Guerrero-Ramos emphasised that it is not possible to conduct comparative randomised trials of radical cystectomy versus bladder-sparing systemic therapies because of the variability in the treatment approaches, and all attempts to do this so far have failed, as “patients do not want a computer to decide their fate.” Guerrero-Ramos highlighted that RCTs are needed to compare different treatments for papillary-only NMIBC, as the FDA is not willing to approve any of these drugs based on single-arm trials. Efforts are also required to learn how to sequence drugs and how to combine them in higher-risk patients.
Guerrero-Ramos summarised that the results of Cohort 2 and Cohort 4 in SunRISe-1 showed that Gem-iDRS has clinically meaningful response rates and is well tolerated.
Guerrero-Ramos concluded: “The future is very exciting for bladder cancer research and treatment.”