Meeting Summary
Millions of Americans live with COPD, a leading cause of morbidity, mortality, and cardiopulmonary events. There is also evidence linking COPD, exacerbations and the increased risk of cardiopulmonary events. Despite these findings, many patients with COPD may be unrecognized or undertreated. At the 2025 American Thoracic Society (ATS) International Conference, pulmonologist Sanjay Sethi from the University at Buffalo, The State University of New York, USA, discussed clinical trials and real-world studies supporting the use of triple therapy containing the long-acting β2-agonist (LABA), formoterol fumarate (FORM), the long-acting muscarinic antagonist (LAMA), glycopyrrolate (GLY), and budesonide (BUD), an inhaled corticosteroid (ICS; BUD/GLY/FORM), delivered as a fixed dose combination via a pressurized metered-dose inhaler (MDI). RCTs of BUD/GLY/FORM involving symptomatic patients with moderate-to-very severe COPD include ETHOS, where patients were required to have ≥1 moderate-to-severe exacerbation in the previous year, and KRONOS, where such prior exacerbation history was not required. In ETHOS, moderate-to-severe COPD exacerbations over 52 weeks were both lower with BUD/GLY/FORM compared with GLY/FORM and BUD/FORM MDIs. All-cause mortality was also assessed as a pre-defined secondary endpoint. In KRONOS, forced expiratory volume in 1 second (FEV1) area under the curve from 0–4 hours (AUC0–4) improved with BUD/GLY/FORM, and was significantly different at Week 24 compared to a BUD/FORM MDI and an improvement in change from baseline in morning pre-dose trough FEV1 versus GLY/FORM. Most common adverse reactions (incidence ≥2%) were upper respiratory tract infection, pneumonia, back pain, dysphonia, oral candidiasis, influenza, muscle spasms, urinary tract infection, cough, sinusitis, and diarrhea. Real-world studies also evaluated the timing of initiation of BUD/GLY/FORM in rates of moderate-to-severe exacerbations and in healthcare resource utilization.
Introduction
Around 14.2 million American adults are living with diagnosed COPD,1 with potentially many more being undiagnosed, noted Sethi at a presentation during the 2025 ATS International Conference. Daily in the USA, COPD was the cause for over 2,500 emergency department visits and over 900 hospitalizations, in 2020.2 As COPD exacerbation history and severity were associated with future exacerbation risk,3 Sethi discussed how “maybe we should think about intervening in those highly symptomatic patients earlier, before their first exacerbation.”
COPD is a leading cause of mortality in the USA,4 accounting for approximately 380 deaths per day in 2021,5 and is projected to remain a leading cause of death in 2050.6 A retrospective cohort study found an association between COPD exacerbations and the risk of all-cause mortality, with the highest risk of death in the month following an exacerbation. However, mortality risk remained elevated for up to 2 years following an exacerbation compared to patients with COPD who have not experienced exacerbations.7 One study found that more than a quarter of patients hospitalized for a severe exacerbation died within 1 year of discharge.8
Evidence is also growing that COPD exacerbations may increase the risk of CV events.9 Post-hoc analysis of a large clinical trial (N=16,485) examining patients with COPD and CV disease or CV disease risk factors found that, in the 30 days following a moderate or severe COPD exacerbation, there was around a four-fold greater risk of a CV event, including CV death, myocardial infarction, stroke, unstable angina, and transient ischemic attack, compared to before a moderate or severe COPD exacerbation.10 CV events are linked to early death, both independently and in conjunction with COPD.9,10 “Clearly,” said Sethi, “a COPD exacerbation is a major event with serious short-term and long-term consequences.”
Triple Therapy in COPD
According to the current Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations, appropriate patients at risk of COPD exacerbation should be treated with triple therapy that includes a LABA, a LAMA, and an ICS.11 One such therapeutic option is the fixed dose combination triple therapy of 160 mcg BUD, 9 mcg GLY, and 4.8 mcg FORM inhalation aerosol.
BUD/GLY/FORM is indicated for the maintenance treatment of patients with COPD. It is not indicated for the relief of acute bronchospasm or for the treatment of asthma.
BUD/GLY/FORM is comprised of a LABA, which acts locally in the lungs as a bronchodilator, a LAMA for bronchodilation, whose effect is predominantly site-specific, and an ICS, which is an anti-inflammatory corticosteroid.12
BUD/GLY/FORM is contraindicated in patients who have a hypersensitivity to BUD, GLY, FORM or other product excipients. It is not indicated for treatment of asthma. LABA monotherapy for asthma is associated with an increased risk of asthma-related death. These findings are considered a class effect of LABA monotherapy. When a LABA is used in fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone. Available data do not suggest an increased risk of death with use of LABA in patients with COPD.12 Additional safety information is available at the end of this article.
Functional Respiratory Imaging Study
Functional respiratory imaging (FRI) utilizes 3D airway models from CT scans of patients with COPD to assess lung deposition of inhaled medicines. Utilizing such a model, a comparison was made between two actuations of BUD/GLY/FORM pMDI, two actuations of BDP/GLY/FORM pMDI (results not shown), and one actuation of FF/UMEC/VI (in a dry powder inhaler). This study assessed lung deposition of these combinations using in silico FRI from 20 patients with moderate-to-very severe COPD (55% male; mean age: 64.9 years; mean [SD] FEV1: 1.3 L [0.5]; mean [SD] percent predicted FEV1: 47.4% [15.9]).13
There are no head-to-head comparisons of the in vivo lung deposition of currently approved fixed dose combination triple therapies for COPD. Data do not imply superior efficacy or safety of one drug over the other. Data are descriptive only.
In the first phase, simulations were performed under the following conditions: 30 L/min mean flow rate, 32.4 L/min peak inspiratory flow, 5 seconds total inhalation time, and 2.5 L total inhaled volume. The delivered dose modeling results are shown in Figure 1. In the second phase, simulations were performed at a mean flow rate of 60 L/min (data not shown).13
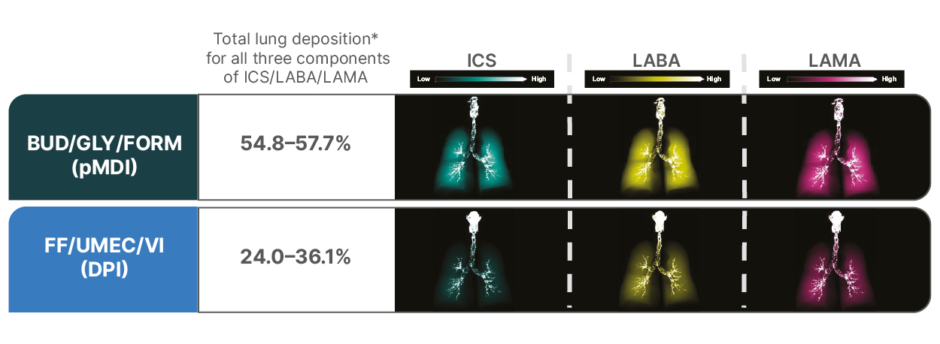
Figure 1: Representative deposition visualization (% delivered dose) by ingredient in a single participant using a 3D airway model (30 L/min flow rate).13
There are no head-to-head comparisons of the in vivo lung deposition of currently approved fixed dose combination triple therapies for COPD. Data do not imply superior efficacy or safety of one drug over the other. Data are descriptive only.
*Total large airway and small airway lung deposition as a percentage of delivered dose.
Adapted from Singh et al.13
BUD/GLY/FORM: budesonide/glycopyrrolate/formoterol fumarate; DPI: dry powder inhaler; FF/UMEC/VI: fluticasone furoate/umeclidinium/vilanterol; ICS: inhaled corticosteroid; LABA: long-acting β2-agonist; LAMA: long-acting muscarinic antagonist; pMDI: pressurized metered-dose inhaler.
As with other modeling techniques, FRI has inherent limitations. It cannot fully replicate all factors of the in vivo setting that may influence deposition, and its modeling of deposition within small airways is incomplete because it does not account for the fraction of drug exhaled from them.13
Budesonide/Glycopyrrolate/Formoterol Fumarate Triple Therapy Clinical Trials
Two main Phase III RCTs led to the approval of BUD/GLY/FORM triple therapy in the US: ETHOS14 and KRONOS.15
ETHOS
ETHOS was a 52-week, Phase III, randomized 1:1:1:1, double-blind, multicenter, parallel-group trial. The trial compared twice-daily BUD/GLY/FORM MDI 320/18/9.6 mcg (n=2,157) with two dual therapies: a LAMA (GLY) + LABA (FORM) combination (GLY/FORM MDI 18/9.6 mcg; n=2,143) and an ICS (BUD) + LABA (FORM) combination (BUD/FORM MDI 320/9.6 mcg; n=2,151). The fourth arm, another triple therapy, is not discussed as it is not licensed in the USA. Enrolled patients were 40–80 years old, current or former smokers (≥10 pack-years), with moderate-to-very severe symptomatic COPD while receiving ≤2 inhaled maintenance therapies and had a history of ≥1 moderate or severe exacerbation(s) in the past year. People with current diagnosis of asthma were excluded.14
The primary endpoint was the annual rate of moderate or severe COPD exacerbations. Secondary endpoints included the annual rate of severe COPD exacerbations and time to death (all cause). Moderate exacerbations were defined as those leading to treatment with systemic corticosteroids and/or antibiotics, and severe exacerbations as those resulting in hospitalization or death.14
As can be seen in Figure 2, the estimated annual moderate or severe exacerbation rates with BUD/GLY/FORM were significantly lower than with GLY/FORM or BUD/FORM in the 52-week ETHOS study.14
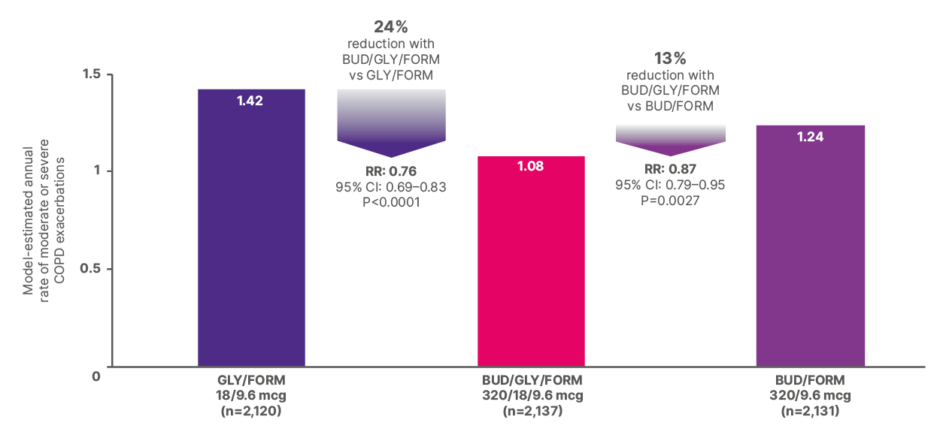
Figure 2: Estimated annual rate of moderate or severe COPD exacerbations in the 52-week ETHOS trial.12,15
BUD: budesonide; FORM: formoterol fumarate; GLY: glycopyrrolate; RR: rate ratio; vs: versus.
Analysis of time to all-cause mortality over 52 weeks was also undertaken in the intent-to-treat population as a pre-specified secondary endpoint (Figure 3). As ETHOS was not designed to assess ICS withdrawal, while the possibility that therapy discontinuation may have contributed to some of the early death events could not be excluded, the findings suggested that the results could not “be explained solely by acute treatment withdrawal.” Due to non-significant results on endpoints higher in the testing hierarchy, these results are observational in nature, and any comparisons between treatment arms should be interpreted with caution.16
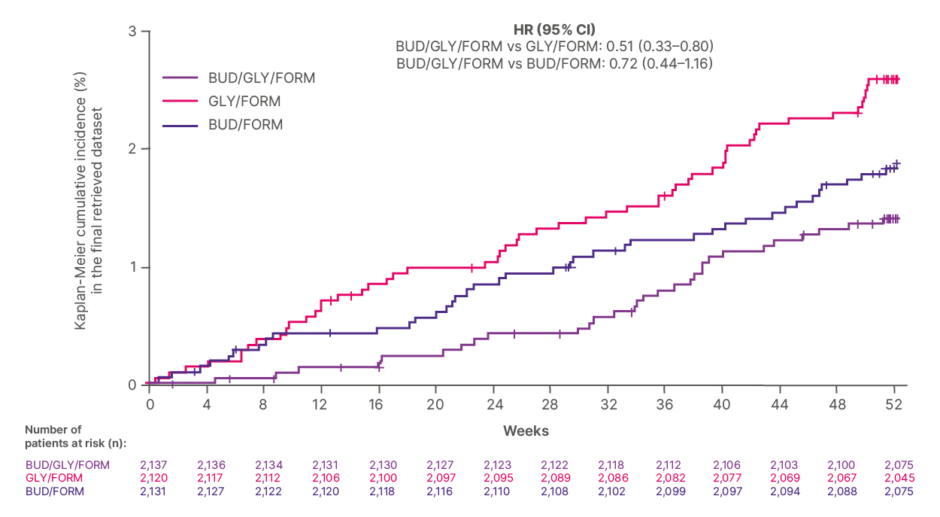
Figure 3: Time to all-cause mortality over 52 weeks in the ETHOS trial.16
Due to nonsignificant results on endpoints higher in the testing hierarchy, these results are observational in nature, and any comparisons between treatment arms should be interpreted with caution. No drug has been proven to reduce all-cause mortality in patients with COPD.
The analysis of time to death from any cause over 52 weeks was performed in the intent-to-treat (ITT) population with the use of a treatment policy estimand, which included all observed data from the patients regardless of whether they continued to receive their assigned treatment. The effect of inhaled corticosteroid withdrawal on these data is unknown.
Adapted from Martinez et al.16
BUD: budesonide; FORM: formoterol fumarate; GLY: glycopyrrolate; HR: Hazard ratio; vs: versus.
KRONOS
KRONOS was a 24-week, Phase III, randomized 2:2:1:1, double-blind, multicenter, parallel-group trial of 1,902 patients with moderate-to-very severe COPD. Twice-daily BUD/GLY/FORM 320/18/9.6 mcg (n=640) was compared with GLY/FORM MDI 18/9.6 mcg (n=627), BUD/FORM MDI 320/9.6 mcg (n=316), and open-label BUD/FORM DPI 400/12 mcg (n=319). BUD/FORM DPI was included for regulatory purposes outside the US; however, the results will not be included here. Inclusion and exclusion criteria were similar to ETHOS; however, patients were not required to have any exacerbation in the prior year.15
Primary endpoints were FEV1 AUC0–4 for BUD/GLY/FORM versus BUD/FORM MDI and change from baseline in morning pre-dose trough FEV1 for BUD/GLY/FORM versus GLY/FORM MDI at Week 24. Secondary endpoints included the rate of moderate or severe COPD exacerbations. Moderate-to-severe exacerbations were defined as above.15
BUD/GLY/FORM demonstrated a significant improvement in FEV1 AUC0-4 versus BUD/FORM (116 ml; P<0.0001) and an improvement in change from baseline in morning pre-dose trough FEV1 versus GLY/FORM (13 mL; P=0.2375) at Week 24.15
Another finding of the KRONOS trial was a difference in the model-estimated rate per year of moderate or severe exacerbations with BUD/GLY/FORM. There was a non-significant reduction of 18%, compared with BUD/FORM, and a significant reduction of 52% compared with GLY/FORM (rate ratio: 0.48; 95% CI: 0.37–0.64; P<0.001).15 The P value is considered unadjusted due to nonsignificant results higher in the testing hierarchy.
In a post-hoc analysis evaluating the rate of moderate or severe exacerbations as stratified by COPD exacerbation history, for patients who had experienced ≥1 moderate-to-severe exacerbation in the past year, the adjusted annualized rate of moderate-to-severe exacerbations was 1.50 with GLY/FORM and 0.63 with BUD/GLY/FORM (rate ratio: 0.42; 95% CI: 0.26–0.67). Adjusted annualized rate with BUD/FORM was 1.05 (rate ratio: 0.60 versus BUD/GLY/FORM; 95% CI: 0.34–1.08). For patients without such an exacerbation history, adjusted annualized rate with GLY/FORM was 0.80 and with BUD/GLY/FORM 0.41, with a rate ratio between them of 0.52 (95% CI: 0.37–0.72). Adjusted annualized rate with BUD/FORM was 0.42 (rate ratio: 0.98 versus BUD/GLY/FORM; 95% CI: 0.63–1.54).17
Also examined post hoc were patients who were symptomatic on ICS/LABA prior to study start and then switched to BUD/GLY/FORM or GLY/FORM. Here, the model-estimated exacerbation rate over 24 weeks, per year among patients with no recent exacerbations, was 0.76 for those who switched to GLY/FORM and 0.43 with BUD/GLY/FORM (rate ratio: 0.57; 95% CI: 0.35–0.94). The post hoc analyses are descriptive in nature and definitive conclusions may not be drawn from them.18
SAFETY DATA
Table 1 shows adverse events in ETHOS in ≥2% of patients.12 Additional adverse reactions that occurred in KRONOS at an incidence of ≥2% included dysphonia (3.3%) and muscle spasms (3.3%).1
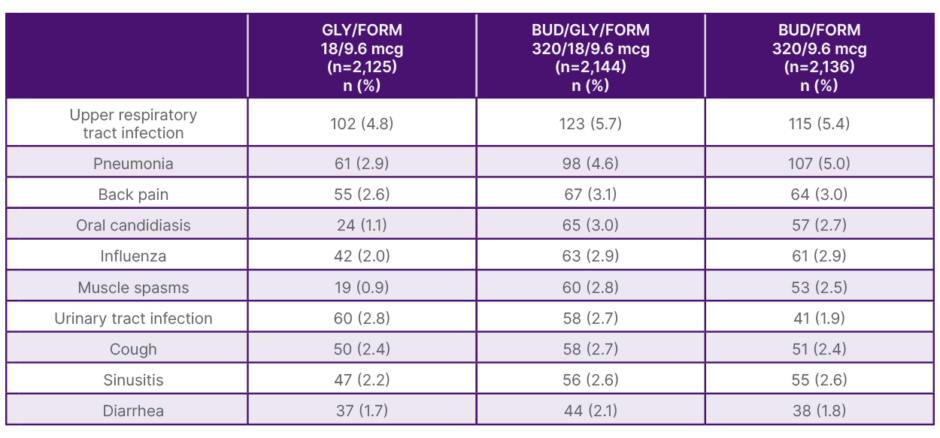
Table 1: Adverse events occurring at an incidence of ≥2% of patients in ETHOS.12
All treatments were administered twice daily.
BUD: budesonide; FORM: formoterol fumarate; GLY: glycopyrrolate.
Real-World Studies of Budesonide/Glycopyrrolate/Formoterol Fumarate Triple Therapy
Additional real-world evidence for BUD/GLY/FORM may “provide another perspective to complement RCTs,” according to Sethi. The EROS Study was a retrospective observational analysis that used US claims data from patients >40 years old (on the index exacerbation date) who initiated triple therapy of BUD/GLY/FORM after ≥2 moderate exacerbations, ≥1 severe exacerbation, or ≥1 moderate exacerbation while on other maintenance treatment in the prior year. The timing of the initiation of triple therapy from the index exacerbation was classified as prompt if it was within 30 days, delayed if between 31 and 180 days, and very delayed if between 181 and 365 days. The primary outcome assessed was the rate of exacerbations per person per year during the variable length post-index period, stratified by the timing of post-index BUD/GLY/FORM initiation. Moderate exacerbations were defined as an outpatient visit with a COPD diagnosis followed by a filled prescription for a short course of oral corticosteroids or antibiotics (≤14 days) within ±7 days of the outpatient visit, or any outpatient visit with a diagnosis of COPD and a corticosteroid injection. Severe exacerbations were defined as an inpatient hospitalization with a primary diagnosis for COPD, or a primary diagnosis of respiratory failure and a second secondary diagnosis of COPD.19 This analysis showed that for patients initiated on BUD/GLY within 30 days of an exacerbation (n=434), the annualized exacerbation rate (95% CI) per patient-year was 1.52 (1.39–1.66). This was 2.00 (1.92–2.09) where therapy initiation was delayed by 31–180 days (n=1,187; relative difference to prompt initiation: rate ratio 0.76 [95% CI: 0.69–0.83]), and 2.30 (2.20–2.40) where the delay was 181–365 days (n=788; relative difference to prompt initiation: rate ratio 0.66 [95% CI: 0.60–0.72]).19
This study should be considered in light of the following limitations. Multivariate regression was used to control for baseline patient demographics and clinical characteristics and this adjustment was limited to variables that are able to be measured within administrative claims. Administrative claims data are subject to data coding limitations and data entry errors by providers, which may result in missing or erroneous data leading to misclassification bias. Immortal time bias is built into the study design, as only those patients who present continuous enrollment and an absence of death following the index exacerbation are eligible for inclusion in the study. Also, while the sample size for this analysis is robust, there may be populations where these results are not generalizable. Lastly, the data is descriptive in nature and definitive conclusions may not be drawn from it.19
How BUD/GLY/FORM use can impact healthcare costs has also been examined. The ARCTOS study was a retrospective analysis of USA claims data following BUD/GLY/FORM initiation. This evaluated 461 patients who were >40 years of age on the index date, had ≥1 inpatient or ≥2 outpatient claims with a COPD diagnosis, ≥1 severe or ≥2 moderate COPD exacerbations during baseline, prescription claim for BUD/GLY/FORM and ≥1 refill within 60 days and an absence of select respiratory diseases. COPD exacerbation-related healthcare resource utilization (HCRU) was calculated based on COPD-related medical and pharmacy services received within the time period of an exacerbation, and the percent change in COPD exacerbation-related HCRU and costs were assessed from the baseline to follow-up.19 Compared with baseline values of 13%, the proportion of patients with COPD exacerbation-related healthcare resource use was 7.4% following BUD/GLY/FORM initiation.20 There were also differences in the mean number of physician office/clinic visits (from 2.81 at baseline to 2.06 following BUD/GLY/FORM initiation), emergency department visits (1.29 versus 0.98), and prescription fills (not including maintenance therapy; 7.1 versus 5.6).20
Study limitations include factors such as administrative claims data are subject to data coding limitations and data entry error, which may result in missing or erroneous data. The study was limited to patients with Medicare fee-for-service and commercial insurance. The results may not be generalizable to uninsured patients or those with other insurance types. The study also does not include a comparison group that did not receive BUD/GLY/FORM, and therefore the data is only generalizable to those on therapy, and cannot be considered solely applicable to the effect of the medicine. This data is observational in nature and should be interpreted with caution.20
Conclusion
The efficacy and safety of BUD/GLY/FORM triple therapy, Sethi concluded, have been established by clinical trials,14,15 with real-world evidence evaluating its impact on exacerbation-related healthcare resource utilization.20
BREZTRI AEROSPHERE® (budesonide, glycopyrrolate, and formoterol fumarate) HCP INDICATION, LIMITATIONS OF USE, AND IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
- BREZTRI is contraindicated in patients who have a hypersensitivity to budesonide, glycopyrrolate, formoterol fumarate, or product excipients
- BREZTRI is not indicated for treatment of asthma. Long-acting beta2-adrenergic agonist (LABA) monotherapy for asthma is associated with an increased risk of asthma-related death. These findings are considered a class effect of LABA monotherapy. When a LABA is used in a fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone. Available data do not suggest an increased risk of death with use of LABA in patients with COPD
- BREZTRI should not be initiated in patients with acutely deteriorating COPD, which may be a life-threatening condition
- BREZTRI is NOT a rescue inhaler. Do NOT use to relieve acute symptoms; treat with an inhaled short-acting beta2-agonist
- BREZTRI should not be used more often than recommended; at higher doses than recommended; or in combination with LABA-containing medicines, due to risk of overdose. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs
- Oropharyngeal candidiasis has occurred in patients treated with orally inhaled drug products containing budesonide. Advise patients to rinse their mouths with water without swallowing after inhalation
- Lower respiratory tract infections, including pneumonia, have been reported following ICS. Physicians should remain vigilant for the possible development of pneumonia in patients with COPD as the clinical features of pneumonia and exacerbations frequently overlap
- Due to possible immunosuppression, potential worsening of infections could occur. Use with caution. A more serious or fatal course of chickenpox or measles can occur in susceptible patients
- Particular care is needed for patients transferred from systemic corticosteroids to ICS because deaths due to adrenal insufficiency have occurred in patients during and after transfer. Taper patients slowly from systemic corticosteroids if transferring to BREZTRI
- Hypercorticism and adrenal suppression may occur with regular or very high dosage in susceptible individuals. If such changes occur, consider appropriate therapy
- Caution should be exercised when considering the coadministration of BREZTRI with long-term ketoconazole and other known strong CYP3A4 Inhibitors. Adverse effects related to increased systemic exposure to budesonide may occur
- If paradoxical bronchospasm occurs, discontinue BREZTRI immediately and institute alternative therapy
- Anaphylaxis and other hypersensitivity reactions (eg, angioedema, urticaria or rash) have been reported. Discontinue and consider alternative therapy
- Use caution in patients with cardiovascular disorders, especially coronary insufficiency, as formoterol fumarate can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, and also cardiac arrhythmias, such as supraventricular tachycardia and extrasystoles
- Decreases in bone mineral density have been observed with long-term administration of ICS. Assess initially and periodically thereafter in patients at high risk for decreased bone mineral content
- Glaucoma and cataracts may occur with long-term use of ICS. Worsening of narrow-angle glaucoma may occur, so use with caution. Consider referral to an ophthalmologist in patients who develop ocular symptoms or use BREZTRI long term. Instruct patients to contact a healthcare provider immediately if symptoms occur
- Worsening of urinary retention may occur. Use with caution in patients with prostatic hyperplasia or bladder-neck obstruction. Instruct patients to contact a healthcare provider immediately if symptoms occur
- Use caution in patients with convulsive disorders, thyrotoxicosis, diabetes mellitus, and ketoacidosis or unusually responsive to sympathomimetic amines
- Be alert to hypokalemia or hyperglycemia
- Most common adverse reactions in a 52-week trial (incidence ≥ 2%) were upper respiratory tract infection (5.7%), pneumonia (4.6%), back pain (3.1%), oral candidiasis (3.0%), influenza (2.9%), muscle spasms (2.8%), urinary tract infection (2.7%), cough (2.7%), sinusitis (2.6%), and diarrhea (2.1%). In a 24-week trial, adverse reactions (incidence ≥ 2%) were dysphonia (3.1%) and muscle spasms (3.3%)
- BREZTRI should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors and tricyclic antidepressants, as these may potentiate the effect of formoterol fumarate on the cardiovascular system
- BREZTRI should be administered with caution to patients being treated with:
- Strong cytochrome P450 3A4 inhibitors (may cause systemic corticosteroid effects)
- Adrenergic drugs (may potentiate effects of formoterol fumarate)
- Xanthine derivatives, steroids, or non-potassium-sparing diuretics (may potentiate hypokalemia and/or ECG changes)
- Beta-blockers (may block bronchodilatory effects of beta-agonists and produce severe bronchospasm)
- Anticholinergic-containing drugs (may interact additively). Avoid use with BREZTRI
- Use BREZTRI with caution in patients with hepatic impairment, as budesonide and formoterol fumarate systemic exposure may increase. Patients with severe hepatic disease should be closely monitored
Indication
BREZTRI AEROSPHERE is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD).
Limitations of use
Not indicated for the relief of acute bronchospasm or for the treatment of asthma.
Please see full Prescribing Information, including Patient Information.
You may report side effects related to AstraZeneca products
US-104162 Updated 1/26







