Authors: *Francesco Locatelli,¹ Franz J. Legat²
1. Department of Nephrology, Alessandro Manzoni Hospital, Past Director, Lecco, Italy
2. Department of Dermatology, Medical University of Graz, Austria *Correspondence to [email protected]
Disclosure: Legat reports receiving travel support fees from AbbVie, Bayer, Celgene, Galderma, Janssen-Cilag, Leo Pharma, Eli Lilly, Novartis, Pelpharma, and Pfizer; lecture fees from AbbVie, Almirall, Bayer Healthcare, Celgene, and Eli Lilly; and advisory board or consultant fees from Almirall, Celgene, Eli Lilly, Menlo Therapeutics, Novartis, Pfizer, Trevi Therapeutics, and Vifor Pharma. Locatelli has nothing to disclose in relation to the topic.
Acknowledgements: Writing assistance was provided by Dr Eleanor Roberts, Beeline Science Communications, Ltd, London, UK.
Disclaimer: The opinions expressed in this article belong solely to the authors.
Support: Publication of this feature was supported and reviewed by Vifor Pharma.
Received: 04.10.21
Accepted: 04.11.21
Citation: EMJ Nephrology. 2021;9[Suppl 4]:2-10.
Abstract
Chronic kidney disease-associated pruritus (CKD-aP) occurs in over two-thirds of patients undergoing haemodialysis. The aetiology of CKD-aP may be multifactorial, involving immune system and opioid dysregulation, peripheral neuropathy, and toxin deposition. CKD-aP can be extremely bothersome for some patients, affecting health-related quality of life and sleep, as well as increasing the likelihood of hospitalisation, infections, and death. However, CKD-aP is often under-reported by patients, who may not associate the pruritus they are experiencing with their CKD, and under-investigated by healthcare professionals, who may be sceptical of the efficacy of treatments. CKD-aP is diagnosed after careful consideration of all other factors, both medical and medicinal, that may be causing bothersome pruritus, aided by pruritus-specific rating scales, questionnaires, and smartphone apps used by patients to track their symptoms. Basic therapy can include tackling the xerosis often seen in patients with CKD and making sure the haemodialysis procedure is optimised, including efficacy of consumables and dialysis-related medications. Drug treatment for CKD-aP may involve careful use of medications targeting opioid imbalance with µ-opioid antagonists and κ-opioid agonists such as naloxone and difelikefalin, or peripheral neuropathy, with gabapentin or pregabalin. Ultraviolet B treatment may also be effective.
INTRODUCTION
With a prevalence of 9.1% worldwide, chronic kidney disease is estimated to affect approximately 700 million people globally.1 For around 3.2 million of these people with CKD, haemodialysis is necessary (0.041% prevalence) and majority of these patients will experience CKD-aP.1 In fact, analysis of data from the Dialysis Outcomes and Practice Patterns Study (DOPPS), a large, ongoing, international, observational study of patients with CKD, revealed that 67% of the included 23,264 patients on haemodialysis experienced some form of chronic pruritus, with 37% rating it as bothering them ‘moderately’ and 19% rating it as bothering them ‘very much’ or ‘extremely’.2 Other studies have confirmed the prevalence of moderate-to-severe pruritus to be around 45% of patients with CKD undergoing haemodialysis.3,4
CDK-aP is not confined to patients undergoing haemodialysis though.5 Analysis of 5,658 non-dialysis patients with Stage 3−5 CKD found CKD-aP in 37−64% of patients (depending on stage and country), with up to 35% experiencing moderate-to-extreme pruritus.6 This puts the numbers of patients with CDK potentially experiencing CKD-aP into the millions.
The aim of this narrative review is to provide information about CKD-aP prevalence, diagnosis, burden, aetiology, assessment, and treatment, utilising a number of studies of the condition, guidelines, and the practice-based experience of the authors.
DIAGNOSIS
CKD-aP necessitates a differential diagnosis to ascertain that the primary aetiology of pruritis is due to CKD and not a comorbid condition.5,7,8 As there are many reasons a patient may be experiencing an itch, such as other medical conditions and/or medications, assessment of all aspects of the patient’s health, treatments, and lifestyle may necessitate a longer consultation than usual. This is important because the lack of time a patient has with their nephrologist is often the reason they do not discuss that they are experiencing pruritus.9 Undertaking a full differential diagnosis of CKD-aP is not just essential to address CKD-aP symptoms, but also to psychologically reassure a patient that what they are experiencing is not due to another medical condition.
One aspect of CKD-aP that may hamper diagnosis is that while pruritus is always present, location and severity may change, which may mislead the healthcare professional (HCP) into thinking that there are varying causes for pruritus.10,11 Pruritus may also be exacerbated by factors such as heat extremes, exercise, and water exposure.5,7,8 All of these factors may differ both between patients and within each patient over time.10,11
Important in evaluating CKD-aP differential diagnoses is to look for subtle pre-existing or newly developed skin conditions or dermatological diseases such as atopic predisposition or dermatitis, psoriasis, (subclinical) urticaria, scabies, or fungal infections.7,8 CKD can often be accompanied by xerosis, which can itself cause pruritus, even in those with no previous history of the condition.11,12 Xerosis frequently and progressively increases in patients with CKD, especially in those undergoing haemodialysis, associated with further renal function deterioration.11
Differential diagnosis for CKD-aP can also include liver, haematologic, and neuropathic conditions as well as diabetes- or anaemia-associated pruritus.5,13 Treatment of these conditions may help alleviate pruritus for some, though this is not the case for all.14 People with CKD are also often on multiple medications, each of which needs investigating as to whether it could be causing or exacerbating pruritus.5 For example, calcium-channel blockers or angiotensin-converting enzyme inhibitors for hypertension can cause itching.7 As such, it’s important to ask a patient prior to initiation of a new medication about their current level of itchiness.
While pruritus itself does not cause skin manifestations, the ‘itch-scratch-cycle’ describes how pruritus occurrence may lead to somewhat pleasurable scratching of an itch to bring relief but can also damage skin.15 Secondary scratch lesions or itchy skin lesions are induced and eventually chronic prurigo may develop.5 This is a skin disease in its own right (with prurigo nodularis being the nodular form) and is not specific to CKD-aP but can arise from various chronic pruritic skin diseases as well as internal medical, neurological, and psychiatric conditions.15 Thus, when chronic prurigo is noted in a patient with CKD, this does not primarily indicate the reason for their pruritus, but rather reveals the existence of a severe and chronic pruritus with an ongoing itch-scratch-cycle.5 The occurrence of chronic prurigo does not relieve the assessing HCP from thinking about other differential diagnoses. In general, in a patient with chronic pruritus and CKD, CKD-aP should be diagnosed as long as no other reasons can be found.
BURDEN OF CHRONIC KIDNEY DISEASE-ASSOCIATED PRURITUS
Although CKD-aP is a standard complication of CKD, under-reporting and underdiagnosis may historically have been overshadowed by a patient’s main kidney-related problems, as well as a number of comorbidities, with CKD treatment, rightfully, being primarily focused on keeping a patient alive.9 Of note though, the DOPPS study data showed that, for patients extremely bothered by CKD-aP, the mortality hazard ratio (HR) (adjusted for a number of covariates) compared with those who were not bothered by CKD-aP was 1.24 (95% confidence interval: 1.08−1.41). Higher HRs were also shown for deaths related to cardiovascular issues and infection and for hospitalisations. CKD-aP severity was additionally associated with haemodialysis withdrawal in this study (HR: 1.50; 95% confidence interval: 1.05−2.14)2 and in a separate analysis, including over 110,000 patients with end-stage renal disease.3
With progressively improving haemodialysis and new treatments for CKD-related comorbidities, the lives of people with CDK are being significantly extended and health-related quality of life (HRQoL) issues are moving more into view. Moderate-to-severe CKD-aP in particular can present a large HRQoL burden, with increasing itchiness severity associated with lower HRQoL scores on several measures.3 This may be related to distress regarding the itching itself as well as to the skin’s appearance, and can impact factors such as working ability and social interactions.11,12 It is no surprise then that people with CKD-aP are also more likely to experience depression and poor mood along with feelings of frustration and annoyance, being washed-out, and drained.2,4,11,12
One of the most prominent HRQoL factors in CKD-aP is often its huge impact on sleep quality,2,4,11 leaving some patients barely sleeping at night for days, weeks, or months on end. Of note, in the DOPPS analysis, the HR for haemodialysis withdrawal was attenuated, though remained strong when adjusted for sleep quality.16 Poor sleep is a serious factor to consider in those with CKD-aP because, as well as being a burden in itself, it can increase the risk of major cardiovascular disease.16
AETIOLOGY AND PATHOPHYSIOLOGY OF CHRONIC KIDNEY DISEASE-ASSOCIATED PRURITUS
Pruritus as an entity can be due to localised changes in the skin and/or to central itch-related mechanisms as well as an interaction between the two.5 Itch-producing factors, known as pruritogens, include cytokines, prostaglandins, neuropeptides, and proteases that can be released by skin-located keratinocytes, sensory neuronal endings, and peripherally circulating immune system cells. Such pruritogens can activate the main cells involved in detection of sensory stimuli, primary afferent sensory neurons. The axon endings of these cells are located in the skin, with their cell body housed in the spinal cord dorsal root ganglia and trigeminal ganglia. In turn, these neurons project to spinal cord dorsal horn neurons, which can activate projection neurons, modulating and propagating the itch signal to the cerebral cortex.5,17
The aetiology and pathophysiology of CKD-aP is still not clear; however, several factors are proposed to interplay in producing CKD-aP, including immune system and opioid dysregulation, peripheral neuropathy, and toxin deposition (Figure 1).5
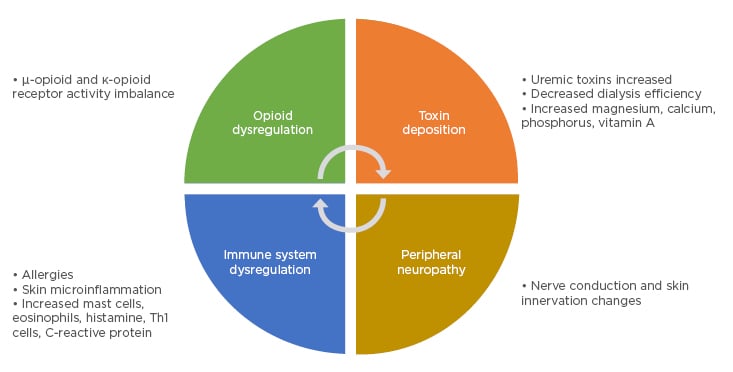
Figure 1: Proposed pathophysiology of chronic kidney disease-associated pruritus.
Adapted from Verduzco and Shirazian.5
One proposal for CKD-aP is neuropathic itching, whereby primary afferent sensory neurons or interneurons are disrupted such that the itch signal is increased.17,18 This may be independent of the presence or signalling by a pruritogen.17 This theory is backed by findings of peripheral sensorimotor neuropathy and dysautonomia. For instance, in one study of 51 patients on haemodialysis, correlations were found between the presence of pruritus and abnormal sympathetic skin response.18 Increased epidermal sensory nerve terminals and fibres have also been shown in patients undergoing haemodialysis; however, this has not been specifically associated with pruritus level.19
In end-stage renal disease especially, immune dysfunction is noted with some aspects up-regulated and some down-regulated.20 Increased inflammatory markers are shown in many patients on haemodialysis, with one study showing significantly higher levels of serum C-reactive protein, IL-6, and Th1 cells in 13 haemodialysis patients with CDK-aP compared to 13 without.21 As such, immune system dysregulation manifesting as systemic and skin microinflammation may play a role in CKD-aP, supported also by a positive response to the use of antibodies to the inflammatory cytokine IL-31 in models of atopic dermatitis.5,22 Additionally, allergy-associated immune cells such as mast cells alongside pruritogens released from these cells such as tryptase, are increased in CKD-aP; however, studies have not been able to correlate mast cell numbers and CKD-aP occurrence.23
Toxin deposition in the skin has been a particular field of interest in people with CKD-aP with ‘uremic toxins’, including calcium, phosphorus, and magnesium, thought to exacerbate pruritus.5 Indeed, in a study of the Japanese cohort from the DOPPS (n=6,480), the likelihood of experiencing moderate-to-extreme pruritus versus no or mild pruritus was significantly linked with higher serum calcium, phosphorus, and calcium–phosphorus product as well as with parathyroid hormone (PTH) levels.4 Regarding the latter, some studies have found parathyroidectomy in haemodialysis patients with secondary hyperparathyroidism can significantly decrease itching.24
However, neither wider analysis of all the DOPPS data or a longitudinal analysis of patients with CKD found such associations with PTH or between various uremic toxins.11,12 For instance, in a DOPPS analysis of 6,256 patients undergoing haemodialysis, mean values for PTH were 355 pg/mL for those without CKD-aP and 322−349 pg/mL for those who experienced mild-to-extreme CKD-aP, with no escalation due to severity. Similarly, mean phosphorus levels were 4.9 mg/dL for patients with non-CKD-aP and 5.0−5.1 mg/dL for those somewhat-to-extremely bothered by it, and mean calcium levels were 8.9 mg/dL for all patients.12 Notably though, these results could be jeopardised by treatment with calcimimetics and phosphate binders. Even in a study as high quality as the DOPPS, adjusting for everything is impossible and residual confounders can persist.
Interestingly, itch intensity in a number of chronic skin conditions is not necessarily correlated with severity. This has prompted investigation of how biopsychosocial factors can enhance the itch sensation, including factors such as vulnerability, stress, support, the itch-scratch cycle, personality, coping mechanisms, and environmental triggers.25 These factors may play a role in CDK-aP as well; however, this is yet to be fully investigated. Severity of CKD-aP may be associated with age, through increases in older age groups.4,11,12 However, this is also a time when comorbidities, and often numerous accompanying medications, may also increase, so differential diagnosis is especially important in older people with CKD-aP.
Finally, opioid imbalance, both in the brain and skin, may be a key factor in CKD-aP.17,26 This is linked to findings of morphine receptors on some excitatory itch-interneurons, itch occurrence following administration of morphine, and abrogation of itch by specific opioid receptor agonists.17 There are two types of opioid receptors thought to be involved in CKD-aP: κ-opioid receptors in the skin and periphery, which may be antagonised, and µ-opioid receptors in the brain, which may be overstimulated.5,17,27
ASSESSMENT OF CHRONIC KIDNEY DISEASE-ASSOCIATED PRURITUS
A recent survey of 301 nephrologists revealed that the majority agreed there was an unmet need for new guidelines for CKD-aP diagnosis and treatment.28 However, there is no universal guidance document for CKD-aP13 and many nephrologists report that their centre does not have a systematic approach to screening.28 Figure 2 shows a suggested diagnosis and treatment pathway.
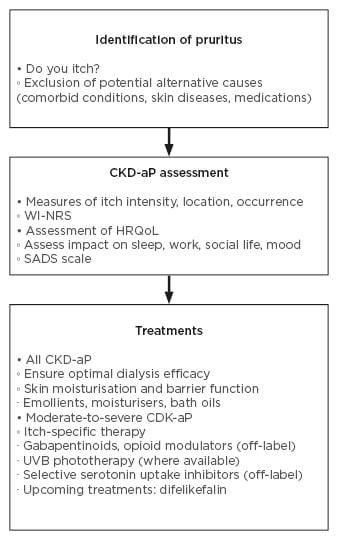
Figure 2: Diagnosis and treatment.2,11,13,29-33
CKD-aP: chronic kidney disease-associated pruritus; HRQoL: health-related quality of life; SADS: Self-Assessed Disease Severity; UVB: ultraviolet B; WI-NRS: Worse-Itch Numeric Rating Scale.
In many practices, CKD-aP is not routinely assessed. In fact, the nephrologist survey revealed that 79% do not use any form of itch scale in clinical practice.28 This may be due to under-reporting by patients, failure of treatment, underestimation by nephrologists, and dismissal of CKD-Ap by HCPs as an important part of CKD.9 For instance, in the DOPPS, 65% of nephrology medical directors underestimated the percentage of their patients on haemodialysis who experienced severe pruritus.12 This analysis also showed that 17% of patients with CKD-aP did not discuss their symptoms with any of their care team or primary care physician/general practitioner.12 One reason for this may be that patients themselves do not connect the pruritus that they are experiencing with having CKD, so do not raise it with their nephrologist. Indeed, one survey found that some patients with CKD only discussed their pruritus with a general practitioner or dermatologist.9 This indicates a need to raise awareness among patients with CKD of CKD-aP with a simple questioning by their nephrologist of whether they are bothered by pruritus.
It is also known that patients may report CKD-aP to only one of their healthcare team, for instance, their nephrologist, a nurse, another haemodialysis staff member, a dermatologist, or a primary care doctor.12 This highlights the need for all HCPs involved in the care of a person with CKD not only to be aware of CKD-aP and discuss it with their patients, but also to discuss it with each other. Under-reporting of CKD-a by patients has also been linked to them not believing it could be treated, as well as this being considered a difficult-to-treat symptom by nephrologists.9
One reason for underassessment of CKD-aP may be because there is no standard way to measure it, with many nephrologists agreeing that a consistent international scale is needed.5,28 This would not only be helpful in general practice but with a globally-defined itch questionnaire used in centres throughout the world, data could be more easily compared.
Assessment of CKD-aP can be carried out using simple visual analogue, verbal, or numeric rating scales that measure severity and can provide a snapshot at a given time or, if repeated, track itch occurrence and intensity over time.5,29,34 While CKD-aP diagnosis and assessment using such scales can take time, for patients undergoing regular haemodialysis that can take many hours, this can be an opportunistic window to complete rating scales. However, time is often limited for HCPs involved in haemodialysis and basic assessment can be carried out with simple tools such as the Worst-Itch Numeric Rating Scale (WI-NRS), developed for patients with psoriatic disease, which asks a patient to rate on a scale of 0−10 ‘the worst itch you felt in the previous 24 hours’.30
Assessment should also be carried out for HRQoL, for instance with use of the Self-Assessed Disease Severity (SADS) scale. Here, patients rate ‘do not have’, ‘sometimes have’, or ‘often have’ scratch marks on their skin and sleep problems associated with itching, and whether itching ‘does not’, ‘sometimes’, or ‘often’ makes them feel agitated or sad. This allows categorisation into mild (none of these problems), moderate (sometimes experiencing these problems), and severe (often experience these problems) levels.29 Another tool, the Brief Itching Inventory, includes a figure onto which patients can draw the location(s) of their itch during the last 24 hours and asks whether itching has interfered with mood, normal work, relations with other people, sleep, and enjoyment of life. Each factor is rated 0−10 (‘itch does not interfere’ to ‘itch completely interferes’).11
In clinical trials, itch has been assessed using more involved indices that assess type, location, and severity of CKD-aP, and how it affects a person’s HRQoL.5 These include the 5-D Itching Scale;35 the Itch Severity Scale (ISS),36 which investigates itch frequency, duration, intensity, pattern, sensation, and symptoms; the Dermatology Life Quality Index (DLQI);37 and Skindex,38 which evaluates HRQoL factors such as emotional, physical, social, cognitive, and psychological effects of pruritus.11
With the advent of e-medicine, there is also the possibility of using itch-tracking apps on smartphones. These can be used by the patient and reviewed by the treatment centre to gain a more dynamic view of itch occurrence and how much a patient is bothered by it over time.39
CURRENT AND EMERGING TREATMENT OPTIONS
Occurrence and severity of CKD-aP can differ from patient to patient so treatment needs to be personalised to need, including managing expectations of what current treatments can offer.
As the majority of people with CKD-aP experience moderate-to-severe xerosis,6,12 topical treatment with regular moisturising cream is important in these cases; an easily accessible step that may help alleviate itching and reduce the level of bothersomness of CKD-aP.5 Itch-relieving ingredients, such as menthol or topical local anaesthetics, may also be useful in an emollient cream.8 Topical treatment with µ-opioid receptor blockers, such as an emollient-based formulation of naltrexone, have been shown to reduce pruritus in people with a number of pruritic skin disorders.40 Hydration may also help tackle xerosis; however, many with CKD and all patients on haemodialysis should restrict their water intake to avoid hypertension and overhydration, including pulmonary oedema.
CKD-aP has also been related to the haemodialysis process, so, one of the first actions when a patient experiences chronic itching is to check haemodialysis efficacy, all consumables, and dialysis-related medications including heparin, the dialysers, the lines, the sterilisation type, and the dialysate concentrate.5 With the more recent modalities of haemodialysis, including high flux, haemodiafiltration, and expanded haemodialysis that can remove larger molecules, pruritus associated with this process could potentially lessen.
An antihistamine may be seen as an obvious choice for treating pruritus and indeed, this is often the first medication used;4,12 however, for many with CKD-aP, such drugs may help only short-term or not at all,5 and such treatment is only recommended for those with urticaria.7,8 Lack of efficacy of antihistamines for CKD-aP may be due to findings that while histamine is a primary pruritogen, there are also many more such molecules released in people with chronic itch.17
Treatment of pruritus due to peripheral neuropathy can include the use of gabapentin or pregabalin, to great effect in some.7,8,41-43 However, side effects such as dizziness and drowsiness may limit their use,41,42 and it is advised to start at a low dose and very gradually taper up.
Much success has been had using κ-opioid agonists; difelikefalin has recently been approved for use in moderate-to-severe pruritus in patients on haemodialysis in the USA, and is currently undergoing European Union (EU) approval.44 In a placebo-controlled, Phase III trial, 51% of 158 patients receiving difelikefalin for 12 weeks had a decrease of ≥3 points on the WI-NRS score, compared with 31% of 165 in the placebo group. As measured by the 5-D itch and Skindex-10 scales, difelikefalin also led to significant improvements in HRQoL. While there were some gastrointestinal-associated side effects, these were mostly transient.33 These results have been confirmed in open label studies.45-47
Another κ-opioid agonist, nalfurafine, has been used in some people with CKD-aP, though it is currently only licensed in Japan.43,48 Nalbuphine, a µ-opioid antagonist and κ-opioid agonist, has also been investigated in patients with CKD-aP, with one study of participants with moderate-to-severe CKD-aP showing a significant decline in itch intensity compared to placebo following 8 weeks of treatment.49 An opioid receptor antagonist, such as naloxone or naltrexone, can also help some, though these are off-label and only used when all other treatments don’t work, as side effects can include dizziness, drowsiness, vertigo, bad dreams, and falls.5,7,8
Another avenue of research is into monoclonal antibodies that target inflammatory factors with potential importance in chronic pruritus. A study investigating the effects of the IL-31 receptor A-targeting agent nemolizumab on prurigo nodularis, a subtype of chronic prurigo in some people with CKD-aP, found subcutaneous administration led to significant decreases in pruritus scores compared to placebo.50 A case report also showed the successful treatment of CKD-aP with dupilumab, which blocks signalling via IL-4 and IL-13 receptors.51 Thus, ILs -4, -13, and -31 may be targets in CKD-aP.
Interestingly, perhaps controversially, advances have also been made in the use of placebo and psychological treatments for chronic pruritus. As at least some proportion of pruritus is centrally processed, this can be influenced to a degree by suggestion of relief.52
Ultraviolet B treatment, as carried out in a phototherapy centre, is a third-line option for CDK-aP.5,8 Where available, it can be administered on the same day as haemodialysis for reducing the burden of appointment attendance for patients. Phototherapy with ultraviolet B is thought to work by interacting with and affecting sensory nerve mediators in epidermal and dermal layers, and resident and infiltrating immune system cells. Treatment is also postulated to upregulate a ‘soluble anti-pruritic factor’, which may be a κ-opioid agonist, that can act both locally and systemically.53 However, such treatment centres are limited and this treatment cannot be used long-term for those on a transplant list, as it is contraindicated when taking immunosuppressants.
CONCLUSION
With many millions worldwide experiencing CKD and undergoing haemodialysis, percentages of those with moderate-to-severe CKD-aP are high enough that it should be a concern in all patients with CKD. This merits awareness of the symptoms and differential diagnosis of CKD-aP as well as how to treat the condition. New and upcoming treatments, such as the κ-opioid receptor agonist difelikefalin, may help improve both CKD-aP and HRQoL.

![EMJ Nephrology 9 [Supplement 4] 2021 Feature Image](https://www.emjreviews.com/wp-content/uploads/2021/11/EMJ-Nephrology-9-Supplement-4-2021-Feature-Image-940x563.jpg)






