BACKGROUND AND AIMS
Hereditary angioedema (HAE) is a rare and potentially life-threatening genetic condition, whereby patients present with recurrent episodes of swelling. This affects the subcutaneous and submucosal tissues of the skin as well as mucous membranes such as the respiratory and gastrointestinal tracts.1
HAE is inherited in an autosomal dominant trend, which can be classified based on either the presence or absence of the levels (Type 1) or the function (Type 2) of the C1-esterase inhibitor, thus resulting in uncontrolled plasma kallikrein activity and an excess formation of bradykinin, a vasoactive peptide.2,3
Berotralstat is a highly selective oral kallikrein inhibitor used to treat both Type 1 and Type 2 HAE in patients aged ≥12 years. The efficiency and safety of the drug for prophylaxis of HAE have recently been established following clinical trials. It then became available in the UK via the Early Access to Medicine Scheme (EAMS) in November 2020.4
In this review, the authors compared their regional experience in using berotralstat since the EAMS was launched in November 2020 to the national survey that was presented at the European Academy of Allergy and Clinical Immunology (EAACI) Congress, July 2022.
OUTCOMES FROM THE NATIONAL UK SURVEY
The survey included 54 patients from 12 regional immunology centres within the UK who were treated with berotralstat.
The survey covered a 3-month period before commencing berotralstat, which was repeated at 3- and 6-month intervals following the commencement of berotralstat. The overall results revealed remarkable improvement in attack frequency following the commencement of berotralstat.5 Data collated included previous prophylaxis, the prevalence of attacks, and quality of life scores via the Angioedema Control Test (AECT). The survey results were similar to the trial data; however, there was a higher percentage of patients discontinuing berotralstat because of adverse effects or unsatisfactory outcomes with regard to the efficacy of berotralstat than previously reported.6
OUTCOMES FROM SALFORD REGIONAL IMMUNOLOGY CENTRE
Ten patients were commenced on berotralstat since the launch of the EAMS in November 2020. Most of the patients (80%; n=8) either did not experience any adverse reactions or only mild symptoms that settled within a few days and continued their therapy. Only 20% of patients (n=2) reported unsatisfactory response to the drug and discontinued berotralstat. Regarding adverse reactions, 30% of patients (n=3) experienced mild headaches for a few days, and 20% (n=2) reported symptoms of gastrointestinal issues. Only one patient experienced cramps, nausea, diarrhoea, and vomiting, which settled within a few days.
In regard to previous use of long-term prophylaxis, 60% of patients (n=6) were established on oxandrolone as long-term prophylaxis treatment. Only 20% of patients (n=2) discontinued berotralstat because of an unsatisfactory response to prevent HAE attacks. These individuals restarted on oxandrolone. The rest of the patients (40%; n=2) successfully continued berotralstat and discontinued oxandrolone with no long-term adverse effects.
CONCLUSION
The authors’ experience with berotralstat showed that most of the patients either did not experience any adverse effects or only mild symptoms that settled within a few days and continued berotralstat. Overall, there was a lower incidence of discontinuation because of adverse effects amongst the authors’ cohort. More data from the post-marketing data is required to establish the real-world experience with regard to efficacy and adverse effects.
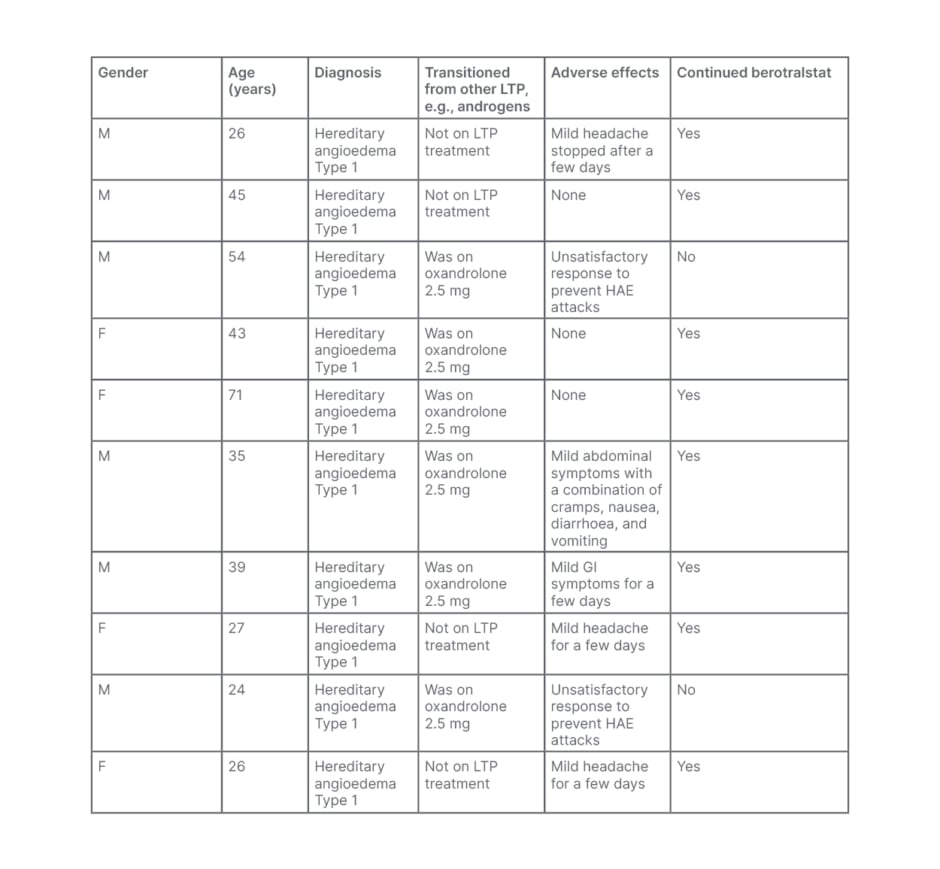
Table 1: Summary of Salford patients with hereditary angioedema who were commenced on berotralstat since the launch of the Early Access to Medicine Scheme (EAMS) in November 2020.
F: female; GI: gastrointestinal; LTP: long-term prophylaxis; HAE: hereditary angioedema; M: male.






