INTRODUCTION
To evaluate the efficacy and safety of silicone-covered metallic ureteral stents (MUS) compared with double-J (D-J) stents in patients with malignantureteral obstruction.1
MATERIALS AND METHODS
This was a prospective, randomised controlled trial. Patients diagnosed with ureteral stricture caused by a malignant tumour with a life expectancy of >3 months and those who had not previously undergone metal ureteral stent (MUS) placement were selected.2-9 Seventy-six ureters (65 patients) were enrolled in this study between January 2020–November 2023. The 76 ureters were randomised 1:1 into the experimental and control groups.10 The experimental group received a covered MUS and the control group received D-J stenting. One ureter in the control group did not undergo stenting because patency was confirmed during retrograde ureterography. Analysis was conducted at 1, 3, and 6 months after the procedure, and the primary endpoint was the primary patency rate determined using CT and diuretic renal scan during the study period. Secondary endpoints included technical success rate, adverse reaction rate, and degree of discomfort caused by the stent.
RESULTS
A total of 76 ureters from 65 patients were enrolled and randomised into two groups: 38 ureters in the experimental group (silicone-covered MUS) and 38 ureters in the control group (D-J stent). There were no statistically significant differences in age, BMI, underlying diseases, and previous treatment histories. However, right-sided ureteral involvement was more common in the experimental group (56.25% versus 27.27%; p=0.023). The experimental group had a higher proportion of right-sided ureteral strictures than the control group (63.16% versus 36.84%; p=0.038; Table 1).
The risk of occlusion within 6 months in the control group was 5.621 times greater than that in the experimental group (95% CI: 1.588–19.899; p=0.0074). Additionally, the log-rank test demonstrated a significant difference in patency survival rates between the two groups (p=0.0024; Figure 1). Stent removal occurred in 7/38 cases (18.42%) in the experimental group and 13/37 cases (35.14%) in the control group (Figure 1). In the experimental group, four stents were removed due to stent-related adverse events (stent migration in two cases, general weakness in one case, and gross haematuria in one case). Three other stents were removed due to worsening hydronephrosis and stent failure. In the control group, all 13 stent removals were attributed to worsening hydronephrosis and stent failure, with no stent-related adverse events reported. Two patients (three ureters) in the control group were excluded from the study because of death from cancer progression during the follow-up period.
Multivariate analysis revealed that D-J stenting was an independent predictor of patency failure (hazard ratio: 6.358; 95% CI: 1.482–27.287; p=0.013; Table 2).
Patient-reported discomfort and quality of life, as measured by the Ureteral Stent Symptom Questionnaire, were compared between the two groups. There were no statistically significant differences between the groups in terms of urinary symptoms, body pain, general health, or work performance at 1, 3, or 6 months after stenting. However, the global quality of life scores were significantly lower in the control group at 3 months (p=0.007) and 6 months (p<0.001).
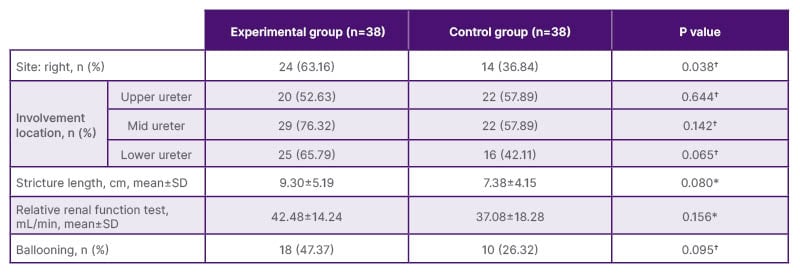
Table 1: Characteristics of ureteral stricture, intent-to-treat analysis.
*Student t test.
†Fisher’s exact test.
SD: standard deviation.
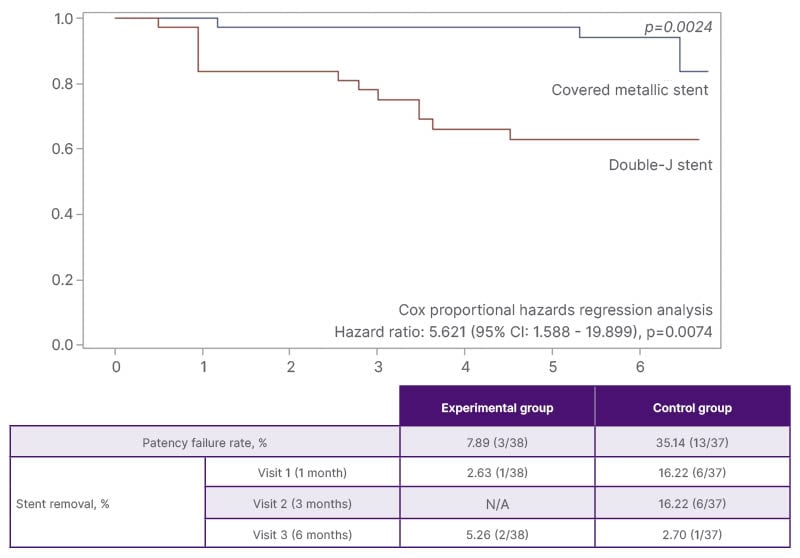
Figure 1: Patency survival rate and risk of occlusion within 6 months.
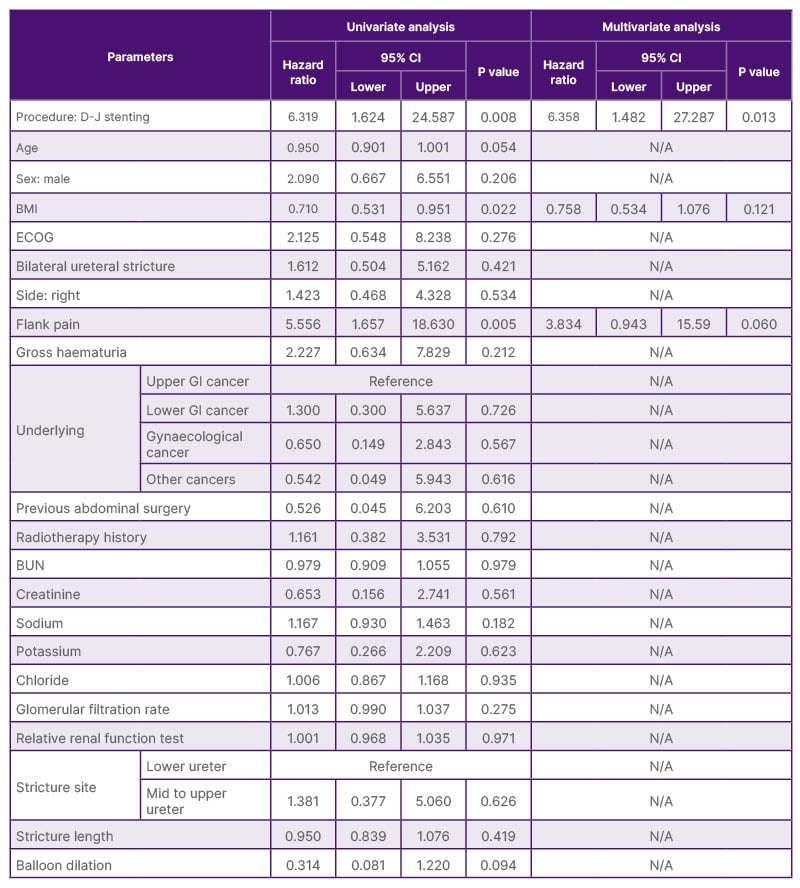
Table 2: Logistic regression analysis for patency failure.
BUN: blood urea nitrogen; D-J: Double-J; ECOG: Eastern Cooperative Oncology Group performance status;
GI: gastrointestinal.
CONCLUSION
The covered metallic stent for patients with malignant ureteral obstruction showed a higher patency maintenance rate than the D-J stent, with no differences in satisfaction and safety.







