Abstract
Urolithiasis is a highly prevalent and multifactorial disease, representing the most common urological condition globally. Its incidence continues to rise, posing a significant public health challenge. Management strategies are tailored to individual patients, considering factors such as stone size, composition, location, and underlying metabolic conditions. This review provides a comprehensive overview of both pharmacological and surgical approaches to urolithiasis, emphasising recent advancements and emerging technologies. Surgical treatments, particularly minimally invasive procedures, have shown considerable improvement, offering highly effective solutions with reduced morbidity. While surgical interventions are essential, pharmacological therapies play a key role in preventing recurrence and addressing metabolic abnormalities that contribute to stone formation. Advances in understanding the molecular mechanisms and pathophysiology of urolithiasis are crucial for developing targeted therapies. A holistic approach that integrates advanced surgical techniques with pharmacological interventions, tailored to individual metabolic profiles, is essential for optimising patient outcomes. Personalised management, supported by regular monitoring of urinary pH, metabolic profiles, and adherence to treatment regimens, is vital to reducing recurrence and improving the quality of life for patients with urolithiasis.
Key Points
1. Urolithiasis is a prevalent systemic disease with a rising incidence. Advances in treatment, including novel therapies, are essential to optimising outcomes and reducing the burden of this condition.
2. This review examines comprehensive management strategies for urolithiasis, including surgical innovations, pharmacological therapies, and non-pharmacological interventions. It highlights the importance of personalised treatment plans and emerging novel therapies.
3. Successful urolithiasis management requires a multifaceted approach, combining tailored surgical, pharmacological, and lifestyle interventions. Emphasising personalised care, novel therapies, and proactive patient management improves outcomes and reduces recurrence rates.
INTRODUCTION
Urolithiasis refers to the formation of stones in the urinary tract and is the most common urological condition globally. It is a multifactorial disease, with both prevalence and incidence increasing worldwide over recent decades. Urolithiasis poses a growing public health challenge, affecting populations across diverse demographics.1 Management strategies are tailored based on stone size, composition, location, and patient-specific factors. This review explores medical and surgical treatments, recent advancements, and innovative technologies, providing a comprehensive overview of urolithiasis management.
OVERVIEW OF MEDICAL (PHARMACOLOGICAL) TREATMENT OF UROLITHIASIS
The management of urolithiasis typically begins with non-invasive approaches that emphasise observation, conservative measures, and medical expulsive therapy (MET). For patients with small stones likely to pass spontaneously, observation is a practical option, particularly when the stones are asymptomatic or cause minimal symptoms. Conservative management strategies aim to alleviate symptoms, facilitate stone expulsion, and prevent recurrence, forming the cornerstone of initial treatment. These strategies are universally applicable, regardless of stone composition, and prioritise non-pharmacological measures such as adequate hydration, dietary adjustments, and lifestyle modifications to promote natural stone passage and reduce recurrence risk.2,3
Adequate hydration is the cornerstone of conservative management, with a recommended daily fluid intake sufficient to achieve a urine output of 2 L or more. This reduces the concentration of stone-forming salts in the urine, supporting the expulsion of smaller stones while preventing new stone formation. Dietary modifications, including sodium restriction to lower urinary calcium excretion, are universally advised, as are adjustments to reduce intake of oxalate-rich foods and animal protein to limit recurrence. Additionally, urinary pH regulation is an important factor in the management of various stone types, as the solubility of certain stone components depends on the acidity or alkalinity of the urine. These general measures provide a strong foundation for managing urolithiasis and are often sufficient in cases without complex underlying conditions.3-6
Pharmacological treatment complements these general strategies and is essential in both acute and chronic urolithiasis management. During acute episodes, the primary goals are to alleviate pain and facilitate stone passage. Pain management is central, with non-steroidal anti-inflammatory drugs (NSAID) being the first-line agents due to their combined analgesic and anti-inflammatory effects. For patients with severe pain or contraindications to NSAIDs, opioids may be used cautiously, while intravenous lidocaine has shown promise as an alternative in select cases.7 Antiemetics such as ondansetron or metoclopramide are frequently used to manage nausea and vomiting associated with acute renal colic.
MET is a pharmacological strategy primarily used for managing ureteral stones measuring 5–10 mm. Alpha-blockers, such as tamsulosin and doxazosin, relax ureteral smooth muscle, facilitating stone passage and reducing the time to expulsion. When combined with anti-inflammatory agents, MET has demonstrated significant efficacy in reducing the need for surgical intervention and improving patient outcomes.3,8 For stones ≤5 mm, the spontaneous passage rate is high, often making conservative management (observation without medication) sufficient. In contrast, MET is particularly effective for distal ureteral stones >5 mm, where spontaneous passage is less likely. However, its benefits are less pronounced for proximal stones due to their lower spontaneous passage rates and higher risk of obstruction. Clinical guidelines recommend considering alpha-blockers as MET for distal ureteral stones >5 mm as part of the treatment options for patients who are amenable to conservative management.3,8
Management of unilateral urological obstruction caused by a ureteral calculus requires a tailored approach based on stone characteristics, urinary tract anatomy, and clinical presentation. Small stones (<5 mm) typically pass spontaneously with conservative management (analgesia, hydration, and MET) for 4–6 weeks. Larger stones or those causing persistent obstruction, severe pain, or renal impairment require surgical intervention.
In cases complicated by infection, such as obstructive pyelonephritis or urosepsis, urgent renal pelvis drainage is necessary to relieve obstruction. Antibiotic therapy should be initiated promptly to control the infection. Definitive stone removal follows once the infection is resolved, and the patient’s condition stabilises.
Surgical intervention is also indicated for complications directly related to the stone or its treatment, such as perforation, avulsions, or infections.
Prompt intervention is essential to prevent further renal damage and manage sepsis-related conditions. Hospitalisation and a combined medical and surgical approach are often required, especially when urosepsis, renal abscess, infected stones, and/or acute renal impairment are present.2,3,8
This approach effectively addresses both the stone and related complications, optimising patient outcomes and minimising long-term renal damage.
Specific Stone Composition Treatments
The treatment of urolithiasis becomes increasingly targeted once the stone composition is identified, as different stone types require distinct therapeutic strategies.
Calcium oxalate stones
Calcium oxalate stones, the most prevalent type, are managed through a combination of pharmacological and dietary measures. Thiazide diuretics are commonly prescribed to lower urinary calcium excretion, thereby reducing the likelihood of stone formation.9-11 Potassium citrate is also used to increase urinary citrate levels, which inhibit stone formation by preventing crystal aggregation. Dietary interventions play a crucial role, focusing on reducing the intake of oxalate-rich foods such as spinach, beets, and nuts while ensuring adequate consumption of calcium-rich foods to minimise oxalate absorption in the gastrointestinal tract.12,13 Additionally, maintaining sufficient hydration is crucial to dilute urinary constituents and reduce the risk of stone formation.
In primary hyperoxaluria (PH), a rare genetic disorder causing excessive oxalate production, standard treatments may not suffice. PH leads to oxalate accumulation in the kidneys, causing recurrent kidney stones, nephrocalcinosis, and progressive renal impairment. As renal function declines and glomerular filtration rate (GFR) falls below 30–40 mL/min/1.73 m², liver oxalate production surpasses renal clearance, leading to systemic oxalate deposition and multi-organ failure.14
In PH1, the most common and severe form of the disorder, pyridoxine (vitamin B6) therapy plays a key role. Pyridoxine can significantly lower urinary oxalate excretion in about one-third of the patients with PH1, and its effectiveness should be assessed through monitoring urinary oxalate levels.14 If pyridoxine proves ineffective, newer therapies such as RNA interference (RNAi) treatments offer promising alternatives. Lumasiran, an RNAi agent targeting glycolate oxidase, has demonstrated a significant reduction in urinary oxalate excretion (65% versus 11%; p<0.001), with 84% of treated patients reaching urinary oxalate levels below 1.5 times the upper reference limit within 6 months.15 Another agent, nedosiran, which inhibits lactate dehydrogenase A (LDHA), a key enzyme in oxalate production, has demonstrated the potential to reduce urinary oxalate levels by approximately 55% in patients with PH1 or PH2, though its efficacy in PH2 remains uncertain.16
Despite these advances, no specific pharmacological treatments are available for PH2 or PH3.
These newer therapies bring hope, particularly for patients in the advanced stages of PH, where renal impairment and systemic oxalosis complicate treatment. In these cases, kidney replacement therapy should be considered before kidney failure develops. Intensified haemodialysis is recommended for patients who lack access to or do not respond to oxalate-lowering therapies.
For patients with PH1 with advanced renal disease (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m²) who fail to respond to pyridoxine therapy, combined liver and kidney transplantation is recommended, regardless of access to RNAi treatments. The decision to perform sequential or simultaneous liver and kidney transplantation should be based on the clinical situation and the surgeon’s preference. However, this may be challenging in low-resource countries due to the limited availability of diagnostic tools, renal replacement therapies, and novel therapies.14 Ongoing research is essential to assess the long-term impact of RNAi therapies and their potential to reduce the need for liver transplantation in patients with multi-organ involvement.
Calcium phosphate stones
Calcium phosphate stones form in alkaline urine and are often associated with underlying metabolic conditions such as renal tubular acidosis. Treatment focuses on correcting hypercalciuria and managing urinary pH. Thiazide diuretics are prescribed to reduce urinary calcium excretion, while potassium citrate or sodium citrate may be cautiously used to balance urinary citrate levels. Monitoring and adjusting urinary pH are essential to prevent excessive alkalinity, which fosters calcium phosphate precipitation.3,6,12
Uric acid stones
Uric acid stones form in acidic urinary conditions and are primarily treated by alkalinising the urine. Potassium citrate or sodium bicarbonate is used to raise urinary pH, enhancing the solubility of uric acid and facilitating its excretion. In cases of hyperuricemia, xanthine oxidase inhibitors, such as allopurinol or febuxostat, are prescribed to reduce uric acid production. It is important to note that uric acid stones can also form in individuals with normal serum and urine uric acid levels but with low urine pH. In such cases, allopurinol and febuxostat have no role in treatment. Regular monitoring of urinary pH is critical, aiming to maintain it within the optimal range of 6.0–6.5 to prevent precipitation of uric acid and other salts.3,6,17
Struvite stones
Struvite stones, commonly associated with urinary tract infection (UTI) caused by urease-producing bacteria, require a multi-faceted treatment approach. The cornerstone of therapy includes antibiotics to eradicate the infection and prevent stone recurrence. Urease inhibitors, such as acetohydroxamic acid (AHA), may also be used to reduce the production of ammonia, which lowers urinary pH and inhibits struvite stone growth. AHA has demonstrated effectiveness in preventing stone growth and delaying recurrence. However, its side effects, including tremulousness and haemolytic anaemia, often limit its use.3,6,17 In some cases, surgical intervention is necessary to remove large or obstructive stones and restore normal urinary function.
Cystine stones
Cystine stones are rare and associated with the hereditary condition cystinuria, which leads to high urinary cystine levels. Treatment strategies emphasise management, primarily involving aggressive hydration to dilute the urine and reduce cystine concentration, often targeting a urine output of at least 3 L/day. Urinary alkalinisation with potassium citrate, targeting a pH of 7.0–7.5, is used to further enhance cystine solubility, and cystine-binding agents such as tiopronin or penicillamine are used for refractory cases. In refractory cases, cystine-binding agents such as tiopronin and D-penicillamine are used, as they form soluble cysteine complexes that facilitate excretion. Measuring free and bound cystine is clinically valuable for adjusting treatment doses, with a target-free cystine level of <100 mmol/mmol creatinine. However, this test is not widely available, and further studies are needed to validate its clinical utility.18 Among cystine-binding drugs, tiopronin is preferred due to its superior efficacy, reducing urinary-free cystine levels approximately 1.5 times more than D-penicillamine, while also increasing soluble mixed disulfide formation.18,19 Unlike D-penicillamine, tiopronin is not excreted in the urine, making the cyanide–nitroprusside test an effective monitoring tool. It is also associated with a lower toxicity profile, leading to fewer treatment discontinuations (31% versus 69% for D-penicillamine in a multi-centre trial).20 In contrast, D-penicillamine, though effective, carries a higher risk of adverse effects, including rash, gastrointestinal symptoms, joint pain, and haematologic complications such as thrombocytopenia and neutropenia. Long-term use may also cause vitamin B6 deficiency, necessitating supplementation. Given its lower toxicity, tiopronin is generally the first choice for patients requiring pharmacological intervention. The dosing and adverse effect profiles of both agents are detailed in Table 1.
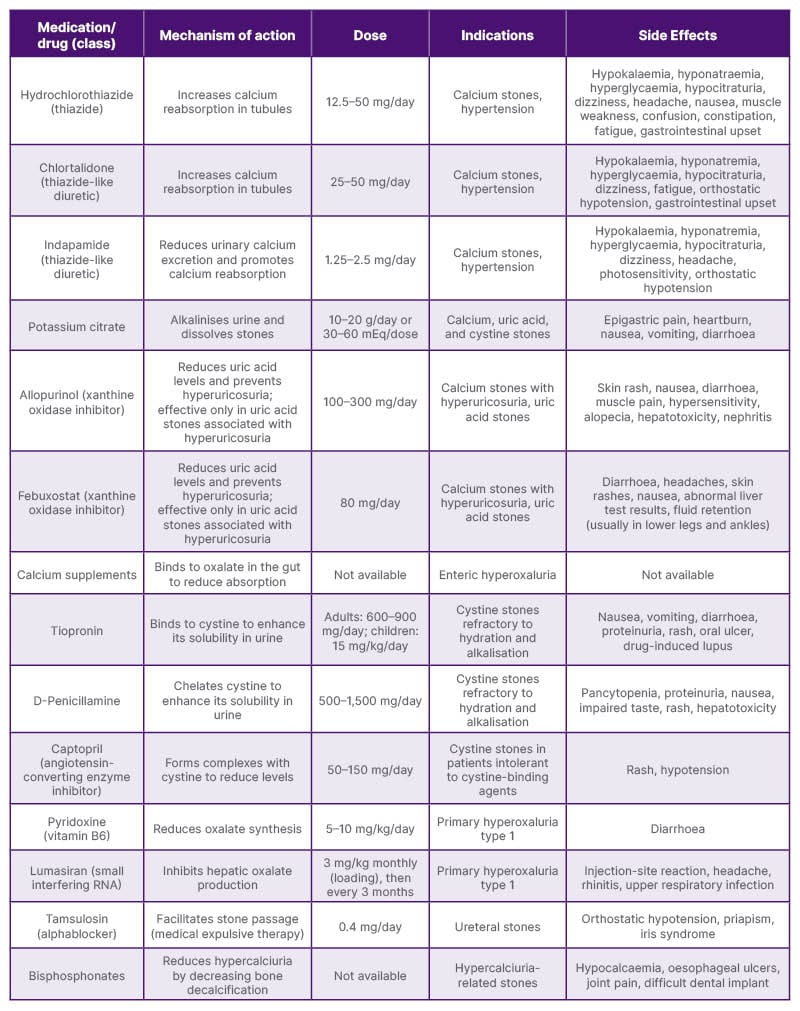
Table 1: Pharmacotherapy for urolithiasis.6,12,13
Regular monitoring of cystine levels in the urine and adherence to these interventions are critical for long-term management. Long-term management of cystinuria requires regular monitoring of urinary cystine levels, blood counts, and urinary protein excretion to optimise treatment and minimise complications.3,6,17,18
Captopril, an angiotensin-converting enzyme (ACE) inhibitor, has been explored for cystine stone treatment due to its sulfhydryl groups, which bind cystine and form a more soluble cystine-captopril complex. While this mechanism theoretically enhances cystine solubility, its clinical efficacy remains controversial. Due to insufficient high-quality evidence, captopril is not recommended as monotherapy but may be considered as an adjunct to tiopronin in patients with high cystine excretion. It is also a potential alternative for those intolerant to tiopronin and D-penicillamine.6,12 Given these considerations, the authors hypothesise that zofenopril, another sulfhydryl-containing ACE inhibitor used for hypertension, could be a potential option for patients with both cystine stones and hypertension. Due to its structural similarity to captopril, it may also enhance cystine solubility while providing anti-hypertensive benefits. However, this hypothesis remains untested, and further research is needed to evaluate its potential role in cystinuria management.
Mixed stones
Mixed stones, such as those comprising calcium oxalate and uric acid or calcium oxalate and phosphate, present unique challenges due to their varied composition. Treatment strategies must address all components of the stone effectively:
Calcium oxalate and uric acid stones: a combination of urinary alkalinisation with potassium citrate to enhance uric acid solubility and the use of thiazides to lower urinary calcium levels is effective. Xanthine oxidase inhibitors such as allopurinol and febuxostat may be added if hyperuricemia is present.
Calcium oxalate and phosphate stones: addressing hypercalciuria with thiazides or thiazide-like diuretics and carefully managing urinary pH to avoid excessive alkalinity are critical.
Urate and phosphate stones: these require precise management of urinary pH to prevent simultaneous precipitation of both components. Regular monitoring of pH ensures the balance needed to prevent further stone formation.8,12,17
In chronic management, pharmacological therapies are tailored to the stone’s composition and the patient’s metabolic profile to prevent recurrence. By addressing the unique metabolic abnormalities associated with mixed stones, clinicians can improve treatment outcomes and reduce recurrence risk.
To support clinical decision-making in pharmacological management, Table 1 provides a comprehensive summary of the available medical therapies for urolithiasis. It highlights the mechanisms of action, recommended dosages, specific indications, and potential side effects for each medication, aiding clinicians in tailoring treatment plans based on stone composition and patient-specific factors.
Innovative Treatments
Emerging therapies in the treatment of urolithiasis are focused on improving patient outcomes through advanced techniques and novel pharmacological interventions. One area of innovation involves the use of nanotechnology to deliver targeted treatments directly to the site of stone formation. These nanoparticles can carry drugs that inhibit crystal growth or dissolve existing stones, offering a minimally invasive alternative to traditional surgical approaches.2,21 Advances in genetic research have also paved the way for personalised medicine, with treatments tailored to an individual’s genetic predisposition to stone formation.2,22 Table 2 provides an overview of the genetic diseases associated with an increased risk of urinary stone formation, emphasising the role of specific genetic factors in stone development. Another promising development is the use of probiotics, such as Oxalobacter formigenes, which degrade oxalates in the gut and reduce urinary oxalate levels. This approach is particularly beneficial for patients with recurrent calcium oxalate stones.13
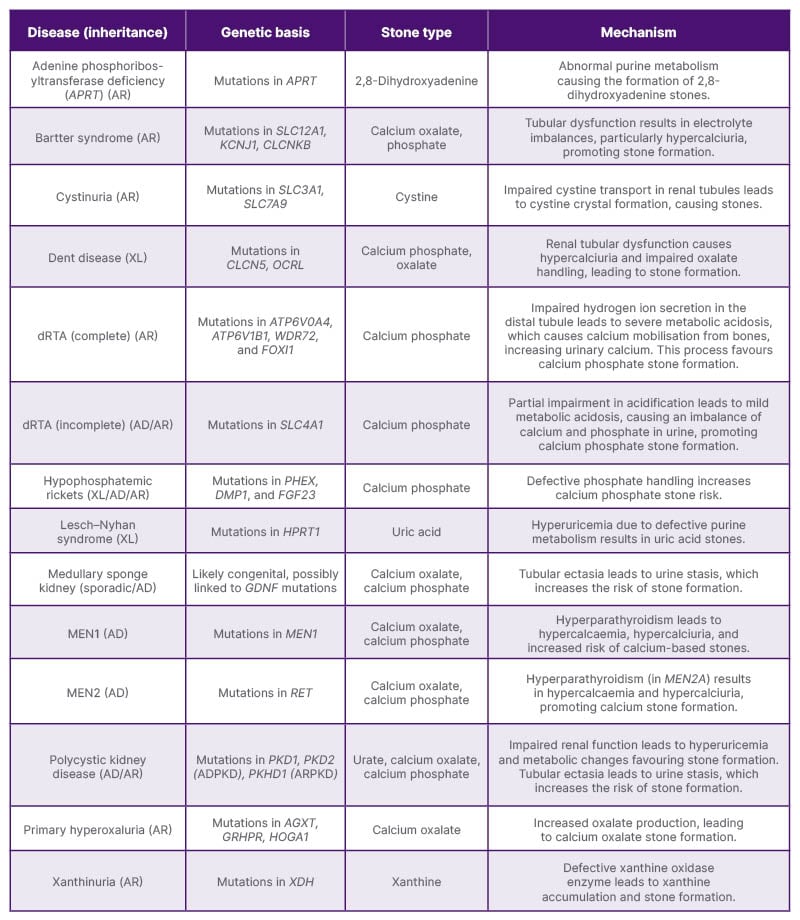
Table 2: Genetic diseases linked to increased urinary stone formation.14,17,18,23-25
AD: autosomal dominant ADPKD: autosomal dominant polycystic kidney disease; AR: autosomal recessive; ARPKD: autosomal recessive polycystic kidney disease; dRTA: distal renal tubular acidosis; XL: X-linked.
Additionally, novel medications targeting specific metabolic pathways involved in stone formation are under investigation, offering the potential to prevent stones at their source.13,26,27 These innovations, while still in varying stages of research and clinical trials, represent the future of urolithiasis management, promising more effective and less-invasive treatment options for patients worldwide.
Dietary and Nutritional Strategies in the Prevention and Management of Urolithiasis
Dietary modifications play a crucial role in both the prevention and management of urolithiasis. Various dietary components, including calcium, oxalate, uric acid, hydration, and micronutrient intake, influence stone formation. Understanding these factors is essential for designing effective dietary interventions.
Hydration and fluid intake
Adequate fluid intake is the cornerstone of urolithiasis prevention. Increasing urine output dilutes stone-forming solutes, reducing their supersaturation. Water is the preferred choice, but certain beverages, such as citrus juices like lemonade and orange juice, may offer additional benefits due to their citrate content, which inhibits stone formation. Evidence suggests that orange juice has a protective effect, while apple and grapefruit juice have not been confirmed as risk factors. However, sugar-sweetened sodas and punch are linked to an increased risk of stone formation. Additionally, increased caffeine intake, particularly above 500 mg/day, has been associated with a lower risk of urolithiasis.28-30
Calcium intake and its role
Dietary calcium plays a paradoxical role in stone formation. While excessive calcium supplementation can contribute to stone formation, an adequate intake of dietary calcium is protective. It binds to oxalate in the intestines, reducing its absorption and urinary excretion. A normal calcium intake of about 1,200 mg/day, combined with a low-salt, low-animal-protein diet, has been shown to reduce the risk of stone recurrence by approximately 50% over 5 years compared to a low-calcium diet of 400 mg/day.31 Dairy products such as milk, yogurt, and cheese are the recommended sources of calcium, while calcium supplements should be used cautiously.28,29
Oxalate consumption and reduction strategies
Oxalate is a major component of calcium oxalate stones, which are the most common type of kidney stone. Foods high in oxalate include spinach, rhubarb, nuts, tea, and chocolate. Reducing dietary oxalate intake, especially in individuals with hyperoxaluria, can help minimise stone risk. Pairing oxalate-rich foods with calcium sources may help reduce oxalate absorption. Oxalate absorption is variable, and in cases of low-calcium diets, increased intestinal absorption of oxalate can contribute to stone formation.28,29
Uric acid and protein intake
Excessive consumption of animal proteins, particularly red meat, poultry, and seafood, increases uric acid production, lowers urinary pH, and promotes uric acid stone formation. High-protein diets have been confirmed as risk factors for urolithiasis. Reducing the intake of beef, pork, shellfish, and chicken can help mitigate this risk. Alkalinising agents, such as potassium citrate, may also be beneficial for patients with recurrent uric acid stones.28,29
Sodium and potassium balance
High sodium intake enhances calcium excretion in urine, increasing the risk of stone formation. Reducing dietary sodium, primarily by limiting processed and fast foods, can help maintain a balanced calcium excretion rate. Conversely, potassium-rich foods such as bananas, oranges, and potatoes contribute to stone prevention by enhancing urinary citrate levels. Notably, only potassium citrate, not potassium chloride, has been found to have a protective effect against urolithiasis.28,29
Role of vitamin and mineral supplements
Certain vitamins and minerals influence kidney stone formation. Vitamin C, when consumed in excessive amounts (more than 1 g/day), increases oxalate production, which elevates the risk of calcium oxalate stones, particularly in men. However, vitamin B6 intake has not been associated with an increased risk of stone formation. Vitamin D supplementation has not been conclusively linked to an increased stone risk in men and younger women, although older women may experience a slight increase in risk.28,29 Among metal cations, dietary copper intake has been associated with an increased risk of kidney stones, while manganese intake appears to have a protective effect. Magnesium plays a critical role in reducing stone formation by binding to oxalate in the gastrointestinal tract, decreasing its absorption and subsequent urinary excretion. Additionally, magnesium enhances urinary citrate levels, further preventing calcium-based stone formation. Research suggests that individuals with low magnesium intake may be at a higher risk of developing kidney stones. Magnesium-rich foods include leafy greens such as spinach and kale, nuts such as almonds and cashews, seeds such as pumpkin and flaxseeds, legumes, whole grains, and bananas. Magnesium supplements, such as magnesium citrate and magnesium oxide, may provide additional benefits for patients with recurrent kidney stones, particularly those with low urinary magnesium levels.28,29 However, excessive supplementation should be avoided due to potential gastrointestinal side effects.
Probiotics and herbal products
Probiotics, particularly gut colonisation with O. formigenes, have been linked to lower urinary oxalate levels, potentially reducing stone formation. However, the effectiveness of oxalate-degrading probiotics remains inconclusive.28
Several herbal products have shown potential benefits in reducing stone formation and recurrence. While clinical evidence is still evolving, traditional medicine has used for long certain plants for their diuretic, anti-inflammatory, and crystallisation-inhibiting properties. Phyllanthus niruri (chanca piedra) demonstrates anti-lithogenic properties by inhibiting calcium oxalate crystallisation, promoting stone dissolution, and reducing oxidative stress. Hibiscus sabdariffa contains natural citrate-enhancing compounds that may help prevent stone formation. Additionally, it has mild diuretic effects, increasing urinary volume and reducing stone supersaturation. Orthosiphon stamineus (Java tea), traditionally used in Southeast Asia for urinary tract health, has been found to exhibit diuretic and antioxidant properties that may help reduce stone formation. Urtica dioica (stinging nettle), known for its diuretic and anti-inflammatory effects, may support increased urine flow and decrease urinary crystallisation potential.28 While these herbal products show promise, more randomised clinical trials are needed to confirm their long-term efficacy and safety.
Dietary patterns and lifestyle modifications
Adopting a balanced diet, such as the Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in fruits, vegetables, whole grains, and low-fat dairy, has been associated with a lower risk of kidney stones.32 A vegetarian diet that includes dairy products appears to be the most protective dietary pattern for urolithiasis prevention. High intake of fruits and vegetables contributes to increased urinary citrate excretion and a reduced stone formation risk. Maintaining a healthy weight and engaging in regular physical activity further support stone prevention.
In conclusion, dietary interventions are integral to urolithiasis management. A well-balanced diet that ensures proper hydration, adequate calcium intake, controlled oxalate and uric acid consumption, reduced sodium intake, and increased fruit and vegetable consumption can significantly reduce the risk of stone formation. The available scientific evidence supports a dietary approach that prioritises fluid intake, balanced calcium consumption, moderate protein intake, and increased consumption of plant-based foods.28,29,31,32 Further research into personalised nutrition strategies may enhance the effectiveness of dietary interventions in kidney stone prevention and management.
Overview of Surgical Treatment Options of Urolithiasis
Surgical interventions are a cornerstone in the management of urolithiasis, especially in patients where medical management proves insufficient, stones are particularly large, or complications arise. These procedures vary based on the stone characteristics and patient-specific factors, encompassing several modalities that differ in their indications, success rates, and potential complications.
Extracorporeal shock wave lithotripsy
Extracorporeal shock wave lithotripsy (ESWL) uses high-energy shock waves to fragment stones into smaller pieces, which are then passed naturally through the urinary tract. This method is particularly effective for kidney and proximal ureteral stones less than 2 cm in size.33,34 ESWL is favoured for its non-invasive nature and minimal recovery time. However, it is less effective for dense stones and stones located in the lower pole due to gravity-dependent fragment clearance.35,36 Temporary haematuria and incomplete fragmentation are common complications.37
Clinical outcomes of ESWL vary based on factors such as stone size, location, and patient-specific characteristics. The stone-free rate (SFR) after the first ESWL session ranges from 46.7% to 69%.38,39 Repeated treatments improve success rates significantly, reaching 70.5% to as high as 93%,39,40 highlighting the importance of repeat procedures in achieving optimal outcomes.
Potential contraindications for ESWL include pregnancy, uncorrected bleeding disorders, aortic aneurysms, severe skeletal deformities, and morbid obesity. The effectiveness of shock wave penetration and stone localisation may be compromised due to technical limitations, making alternative modalities such as ureteroscopy (URS) or percutaneous nephrolithotomy more suitable.33
These variations underscore the importance of individualised patient assessment when considering ESWL for stones less than 2 cm. Factors such as stone composition, density, and anatomical considerations can significantly influence treatment outcomes. A thorough evaluation is essential to optimise the efficacy of ESWL and determine the necessity for additional treatment sessions.
Ureteroscopy
URS is a minimally invasive procedure that involves the insertion of a flexible or rigid ureteroscope through the urethra, enabling direct visualisation and fragmentation of stones using laser lithotripsy. This method is highly effective for treating ureteral and renal stones, particularly stones smaller than 1 cm, with SFR exceeding 90%.41,42 Flexible ureteroscopy (FURS) extends the capabilities of URS by allowing access to challenging locations such as the renal calyces and renal pelvis, which are difficult to reach with rigid scopes.
Despite its minimally invasive nature, URS is associated with potential complications, including ureteral injury, perforation, and post-operative strictures. Ureteral avulsion, though rare (0.06–0.45%), is one of the most severe complications of URS, often related to the use of an overly large ureteroscope or attempts to extract an inadequately fragmented or impacted stone from the proximal or mid-ureter. It typically results from excessive stretching at the ureter’s weakest point, which may be further aggravated by factors such as impaired fluid irrigation during laser lithotripsy, weakening the ureter.43,44 Potential contraindications include untreated UTIs and severe ureteral strictures that may prevent safe scope passage. Advances in laser technologies, such as the holmium:yttrium–aluminium–garnet (holmium:YAG) and thulium fiber laser (TFL), have significantly improved the efficacy and safety of URS.29 The holmium:YAG laser, considered the gold standard, effectively fragments stones of all compositions, though it may lead to stone retropulsion. The TFL offers faster ablation rates and reduced retropulsion, making it particularly advantageous in complex cases.45
Percutaneous nephrolithotomy
Percutaneous nephrolithotomy (PCNL) is the gold standard for managing large stones (>2 cm) and complex staghorn calculi. The procedure involves accessing the kidney through a small incision in the back, allowing for direct removal or fragmentation of stones. PCNL achieves high success rates, with SFRs reaching 92% for staghorn calculi.2,46
However, PCNL is not without risks. Common complications include bleeding, infection, and, in rare cases, injury to adjacent organs. Contraindications include active UTIs, severe cardiopulmonary comorbidities, or uncorrected coagulopathies. Recent innovations such as suction-assisted systems and laser integration have enhanced the procedure’s outcomes while minimising complications.2,3 These advancements have made PCNL a reliable option for patients with complex or large stone burdens.
Open and laparoscopic surgery
Open and laparoscopic surgeries are reserved for patients with complex anatomical abnormalities or other cases where minimally invasive methods are not feasible. These approaches allow for precise stone removal but are associated with longer recovery periods and higher morbidity compared to minimally invasive options.2,47
While their use has declined due to advancements in less invasive techniques, open and laparoscopic surgeries remain valuable in specific scenarios. Potential contraindications include severe medical comorbidities that preclude surgical interventions. The success rates for these procedures are high in appropriately selected cases but their risks include post-operative pain, infection, and extended hospital stays.
Advancements and Combination Modalities Advancements in technology
Disposable ureteroscopes have reduced the risk of cross-contamination and eliminated the need for sterilisation, enhancing procedural consistency. Studies demonstrate that disposable and reusable ureteroscopes have comparable SFRs, complication rates, and clinical outcomes. While disposables minimise infection risks and sterilisation needs, they generate significant waste, raising concerns about long-term environmental sustainability. Reusable ureteroscopes, although generating less immediate waste, require extensive cleaning and maintenance, contributing to resource consumption. Despite these compromises, disposables are particularly beneficial in high-volume or resource-limited settings, where infection control and efficient turnover are prioritised.31,35–37
However, in regions such as sub-Saharan Africa, where the majority of patients pay for healthcare services out of their pocket, both the financial burden of procedures and the limited availability of disposable ureteroscopes can hinder their widespread adoption. Despite their advantages in infection control and procedural efficiency, these economic and logistical challenges faced by patients and healthcare providers may limit their feasibility in these settings.42,48–50
Robot-assisted FURS is another notable innovation, offering enhanced surgeon dexterity and precision in complex cases. These robotic systems help reduce surgeon fatigue and improve surgical outcomes.2,51 AI integration into FURS has further revolutionised the field by assisting in real-time stone detection and procedural guidance, which is particularly beneficial for less-experienced surgeons, ultimately improving patient outcomes.2
High-intensity focused ultrasound (HIFU) is an emerging non-invasive technology that is being explored as a potential alternative to conventional ESWL. HIFU uses focused ultrasound waves to fragment stones, offering the advantage of avoiding radiation exposure and eliminating the need for external shock waves. Although still in the experimental phase, HIFU presents a promising treatment modality for certain types of kidney stones, especially in patients who are contraindicated for more invasive procedures.52-54
Recent advances in laser technology, such as the TFL and holmium:YAG laser, have significantly improved the efficiency and safety of URS procedures. The TFL offers faster ablation rates, reduced stone retropulsion, and more precise targeting, which is particularly beneficial in complex stone locations.45 The integration of these advanced lasers has enhanced the success rates of ureteroscopic treatments for both renal and ureteral stones.
Miniaturised percutaneous nephrolithotomy (mini-PCNL) is a less-invasive version of traditional PCNL, utilising smaller instruments and smaller incisions, which result in reduced blood loss, faster recovery, and fewer complications. This technique is particularly effective for large stones while still maintaining high SFRs. Mini-PCNL represents a key step forward in reducing the morbidity of patients undergoing complex stone removal procedures.46
Combination modalities
For complex cases, combining multiple surgical modalities can yield synergistic effects, improving stone clearance and minimising the need for repeated procedures.45 This approach is particularly beneficial for patients with staghorn calculi or other complex stone formations.
Endoscopic combined intrarenal surgery (ECIRS) is a modern, minimally invasive technique for treating large and complex renal stones. This method primarily integrates URS/FURS and PCNL to optimise SFRs. By enabling simultaneous visualisation and manipulation through both transureteral and percutaneous access, ECIRS enhances stone clearance compared to traditional approaches. Additionally, ECIRS may incorporate adjunctive techniques such as ESWL or mini-PCNL to further improve efficacy and patient outcomes.2
By leveraging the strengths of both PCNL and URS/FURS, surgeons can optimise SFRs while minimising patient morbidity.2,42,52 The flexibility of URS allows for precise stone localisation and fragmentation, while percutaneous access provides direct entry into the renal collecting system, facilitating removal of larger stone fragments and reducing intrarenal pressure. This approach lowers the incidence of post-operative sepsis and is particularly advantageous for patients with anatomical anomalies or complex stone burdens. The integration of advanced technologies, such as holmium:YAG laser lithotripsy and digital endoscopes, further enhances procedural success.45
ECIRS is performed under general anaesthesia and requires a multi-disciplinary team, including urologists and anaesthesiologists. Intraoperative ultrasound and fluoroscopy guide precise instrument placement. The supine position minimises anaesthetic risks and shortens operative time by eliminating the need for patient repositioning. Although risks such as bleeding, infection, or injury to adjacent structures exist, they are significantly lower than with traditional methods due to the controlled percutaneous renal access under ultrasound guidance. Additionally, the use of suction-assisted systems enhances stone fragment clearance, further improving procedural outcomes.2,42,45,47
Another innovative combination under research involves pairing HIFU with other modalities such as URS. While still being explored for its clinical efficacy, HIFU has the potential to fragment stones in challenging locations or those less amenable to traditional methods. URS or FURS can then be used to remove the resulting fragments. This multi-modal approach may improve patient outcomes by utilising the strengths of each technique, particularly for patients with stones in hard-to-reach locations or those with multiple, fragmented stones.52-54 Further studies are required to establish its effectiveness and safety in routine clinical practice.
To assist in clinical decision-making, Table 3 provides a clear comparison of the various surgical approaches for urolithiasis treatment, outlining the advantages, complications, outcomes, limitations, and contraindications associated with each modality.
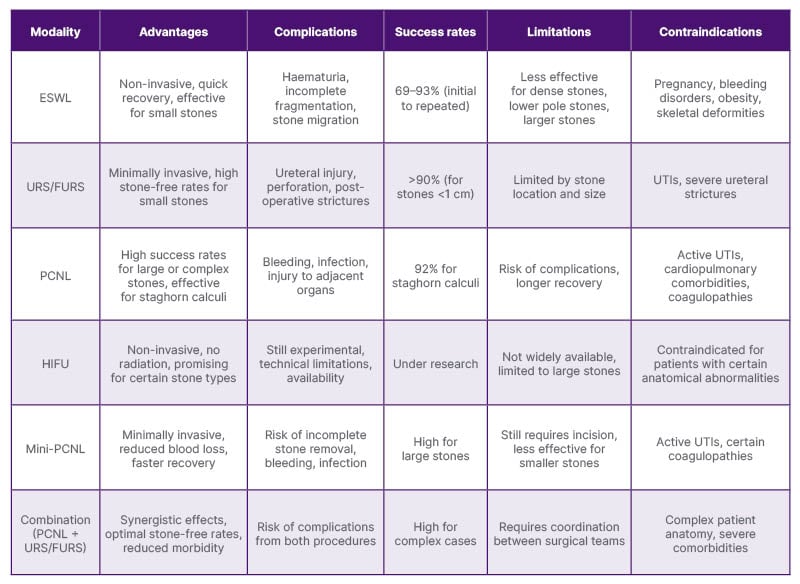
Table 3: Comparison of surgical modalities in urolithiasis treatment: advantages, complications, outcomes, and limitations.
ESWL: extracorporeal shock wave lithotripsy; FURS: flexible ureteroscopy; HIFU: high-intensity focused ultrasound; PCNL: percutaneous nephrolithotomy; URS: ureteroscopy; UTI: urinary tract infections.
CONCLUSION
The management of urolithiasis has undergone substantial advancements in surgical (urologic) treatments, offering minimally invasive and highly effective solutions. However, pharmacological management, crucial for preventing recurrence and addressing metabolic abnormalities, requires further development. A holistic approach integrating surgical innovation with tailored medical interventions is essential to optimising outcomes and mitigating the burden of this disease.
Personalised management guided by regular monitoring of urinary pH, metabolic profiles, and adherence to dietary and pharmacological regimens forms the cornerstone of effective long-term care. Tailoring treatment to specific composition of kidney stones not only improves outcomes but also reduces recurrence, emphasising the importance of sustained patient education and support.
Advances in understanding the pathophysiology of urolithiasis, including molecular pathways such as crystal nucleation and aggregation, as well as genetic predispositions affecting metabolism and stone composition, are opening new avenues for more precise therapies. By integrating surgical expertise, personalised pharmacological care, and proactive monitoring within a comprehensive framework, clinicians can significantly enhance patient outcomes, reduce recurrence rates, and improve the quality of life for individuals affected by urolithiasis.







