Meeting Summary
Prostate cancer is the second most common cancer and the fifth leading cause of cancer death in males worldwide, with over 1.4 million cases and more than 397,000 deaths in 2022. Up to one-third of patients with prostate cancer will develop metastases, which are significantly associated with mortality. Hormone-sensitive prostate cancer that is already metastatic at diagnosis (de novo metastatic hormone-sensitive prostate cancer [mHSPC]) is an aggressive form of the disease that is associated with poor survival outcomes. Loss of function of the tumor suppressor, phosphatase and tensin homolog deleted on chromosome 10 (PTEN), is a key driver in the development of prostate cancer, particularly metastatic disease. Patients with a tumor biomarker of PTEN loss or deficiency have a particularly poor prognosis. This article summarizes a symposium, ‘Pioneering Precision Medicine in Prostate Cancer, The Role of PTEN in Metastatic Hormone-Sensitive Prostate Cancer (mHSPC)’, held at the 2025 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium in San Francisco, California, USA, in February 2025. The article highlights the unmet need in mHSPC, the evolving treatment landscape for this disease, and the poor prognosis linked to PTEN loss or deficiency in de novo mHSPC. The pathways and biomarkers associated with metastatic prostate cancer are explored, with a particular focus on the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/PTEN pathway. The therapeutic rationale for dual targeting of androgen receptor (AR) and AKT pathways, the outcomes of PTEN deficiency in prostate cancer, and PTEN deficiency as a biomarker for prostate cancer, are also discussed. Diagnostic technologies to test for PTEN deficiency and PTEN alterations in metastatic prostate cancer are outlined, and the potential relationship between the extent of PTEN deficiency and the sensitivity of targeted treatment is considered. In addition, topline findings released after the symposium with the AKT inhibitor, capivasertib, in combination with abiraterone and androgen deprivation therapy (ADT) in patients with PTEN-deficient de novo mHSPC in the Phase III study, CAPItello-281, are presented in this article.
Introduction
Prostate cancer is the second most common cancer and the fifth leading cause of cancer death in males worldwide, with over 1.4 million cases and more than 397,000 deaths in 2022.1 Most cases of prostate cancer present at an early stage and often have an indolent course, requiring no or minimal treatment.2 However, up to one-third of patients will develop metastases during their disease course.3 Metastatic prostate cancer is significantly associated with mortality, with approximately 30% of patients surviving 5 years after diagnosis,4 compared with an overall 5-year survival rate of 97.5% for all prostate cancers.5 De novo mHSPC (also known as metastatic castration-sensitive prostate cancer) is an aggressive form of the disease and is associated with poor survival outcomes.6,7 One of the most common driving events in the development of prostate cancer, particularly metastatic disease, is loss of function of the PTEN tumor suppressor, which leads to dysregulated activation of the PI3K signaling network.8,9 Patients with prostate cancer and a tumor biomarker of PTEN loss or deficiency have a particularly poor prognosis.10
The Unmet Need in Metastatic Hormone-sensitive Prostate Cancer
Hormone-sensitive prostate cancer at diagnosis is often localized (i.e., non-mHSPC), but in more aggressive phenotypes, the disease is already metastatic (i.e., mHSPC).11 The presence of metastatic disease at initial prostate cancer diagnosis (synchronous/de novo disease) is associated with a greater risk of progression and worse outcome than metastases that develop after local therapy for prostate cancer (metachronous/recurrent disease), as shown by a shorter time to castration resistance and reduced median overall survival (OS).12,13 Furthermore, PTEN loss or deficiency occurs in approximately one in four patients who present with de novo mHSPC, and is associated with even poorer outcomes.14-17
Despite the advances in mHSPC therapy, progression to metastatic castration-resistant prostate cancer (mCRPC) still occurs, and survival rates in patients with metastatic disease, particularly those with PTEN loss or deficiency, are poor.14 There are no biomarker-driven therapies approved for mHSPC; therefore, the treatment options are provided as part of an “all-comers approach” (standard, non-personalized treatment).12,18
Vice President and Franchise Head, Andrew Foxley, Late Oncology R&D, AstraZeneca, Cambridge, UK, indicated that there is a critical need for new predictive biomarkers, as well as novel, effective, biomarker-driven treatments, and more intensive and tailored treatment strategies for patients with mHSPC, particularly those with PTEN-deficient disease.19,20
The Evolving Treatment Landscape for Metastatic Hormone-sensitive Prostate Cancer
Androgens (male sex hormones), including testosterone, are key drivers in the development of prostate cancer.7,21 Hence, hormone therapies, such as ADT, are used to reduce the levels of androgens in the body; however, resistance to these therapies is common.6,7,22 Clinical studies have been conducted to assess a range of different treatment options in advanced prostate cancer, mHSPC, or mCSPC, including intravenous (IV) ADT,23 IV ADT with chemotherapy,24,25 IV ADT with AR pathway inhibitor (ARPI),26-30 oral ADT,31 and IV ADT with chemotherapy and ARPI (triplet therapy).32,33
The first approved treatment for mHSPC was IV ADT in the 1940s.23 This remained the only available treatment for this disease until the 2010s, when the first ARPIs were approved.34 Current treatment regimens for mHSPC primarily comprise ADT in combination with ARPIs, with or without chemotherapy.12
A treatment paradigm for mHSPC based on the National Comprehensive Cancer Network (NCCN) guidelines is shown in Figure 1.12 The paradigm includes treatment recommendations based on the location of the metastases, the presence or absence of metastases at diagnosis, high- versus low-volume disease, and high- versus low-risk disease.12 The NCCN guidelines recommend ADT in combination with ARPIs and docetaxel for first-line treatment of mHSPC.12
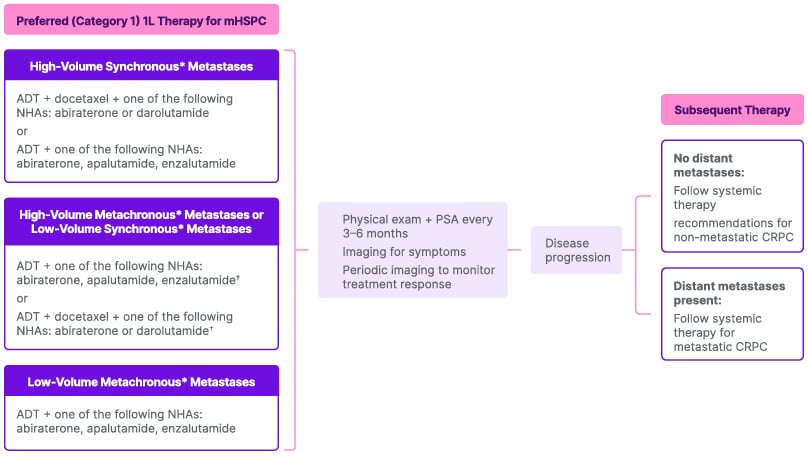
Figure 1: National Comprehensive Cancer Network guidelines: systemic therapy for metastatic hormone-sensitive prostate cancer.12
*Synchronous metastases are those that were discovered upon initial diagnosis; metachronous metastases are those discovered after initial treatment for earlier stage (localized) disease.36
†Another option, although not ‘preferred’ by the NCCN, is ADT plus external beam radiotherapy to the primary tumor for low metastatic burden with or without one of abiraterone or docetaxel.
1L: first line; ADT: androgen deprivation therapy; CRPC: castration-resistant prostate cancer; NCCN: National Comprehensive Cancer Network; NHA: novel hormonal agent; PSA: prostate-specific antigen.
During the symposium, Foxley noted that there does not appear to be a direct link between risk, based on the Gleason grade (Gleason scores assigned range from 6 to 10, with 6 being the lowest grade cancer),35 and PTEN loss or deficiency. In addition, patients with tumors classified as low risk according to standard approaches who have PTEN deficiency have poorer outcomes than those without PTEN-deficient tumors.
Pathways and Biomarkers Associated with Metastatic Prostate Cancer
Metastatic prostate cancer is often characterized by mutations in several biological pathways, including the WNT, P13K/AKT/PTEN, AR, and DNA repair pathways.37-39 The presence of specific biomarkers in a patient with metastatic prostate cancer might influence the type of therapy offered to the patient. Potential therapy choices and the associated biomarkers in mCRPC include poly ADP ribose polymerase inhibitors for homologous recombination repair gene alterations, such as BRCA1/2 mutations; immune checkpoint inhibitors for mismatch repair deficiency and tumor mutational burden-high; lutetium Lu-177 for prostate-specific membrane antigen; and chemotherapy (in certain settings) for AR splice variant 7.12 There are no biomarker-driven therapies approved for mHSPC.12 The search for biomarkers is a growing field of interest in mHSPC, with PTEN being developed as a prognostic biomarker to discern indolent tumors from those that are likely to progress.8
The PI3K/AKT Pathway and PTEN in Prostate Cancer
The PI3K/AKT pathway is one of the most commonly disrupted pathways in cancer cells.40 This pathway is hyperactivated in several cancers, contributing to tumor growth, progression, and development of treatment resistance.40
AKT is a central node and control mechanism in many types of cancer, linking to cell survival through FOX01, cell growth and protein synthesis via mTOR, and cell proliferation by means of GSK3. AKT plays a central role in the PI3K/AKT pathway, modulating a range of substrates involved in cell growth, proliferation, and metabolism, and is a frequent driver of treatment resistance.40
PTEN has a direct relationship to the P13K/AKT pathway, modulating PI3K/AKT signaling by preventing AKT activation.15,16 PTEN function is often lost in prostate cancer. Loss of function of PTEN by deletion or mutation is identified in approximately 20% of primary prostate tumor samples at radical prostatectomy and in up to 50% of castration-resistant tumors.16 Foxley outlined that in cases of PTEN loss or deficiency, AKT activation is no longer suppressed, thereby activating AKT signaling, leading to hyperactivation of the PI3K/AKT/PTEN pathway.41 This results in cell proliferation and tumor growth, worse outcomes, and increased risk of recurrence.41
Foxley explained that PTEN deficiency is like “taking the brake off the AKT pathway”, and no matter how much the AR axis is being “driven down”, a key node of proliferation (i.e., AKT–PTEN) is not being controlled.
Inhibition of Both the Androgen Receptor and PI3K/AKT/PTEN Pathways
There is cross-talk between the AR signaling pathway, which leads to cell differentiation and survival, and the PI3K/AKT/PTEN pathway, which results in cell proliferation and prostate cancer progression through PI3K/AKT/PTEN-dependent signalling.42-45 AR signaling and the PI3K/AKT/PTEN pathway are reciprocally cross-regulated; therefore, inhibition of one leads to upregulation of the other.45 Foxley clarified that inhibiting (“driving down”) the AR axis with hormonal therapy leads to increased reliance of the tumor cell (“an uptick in reliance”) on the PI3K/AKT/PTEN pathway, and that giving AKT inhibitor alone might drive disease because the AR axis is uncontrolled.
Activation of AKT signaling as a result of PTEN deficiency is associated with a reduced benefit from AR pathway blockade.46 Targeting the AR and the PI3K/AKT/PTEN pathways simultaneously might address multiple mechanisms of tumor growth and resistance. Foxley specified: “There is a symbiotic relationship between the AR pathway and the PI3K/AKT/PTEN pathway. Therefore, there are two brakes that are needed in prostate cancer; one on each of these pathways. Implementing the brake on only one of these pathways, particularly in the context of PTEN deficiency, is not going to be effective.”
Outcomes of PTEN Deficiency in Prostate Cancer
PTEN deficiency in prostate cancer is associated with poorer prognosis, and shorter time to progression (recurrence or relapse), progression-free survival (PFS), and OS compared with intact PTEN, as discussed below.
Prognosis
An integrated analysis of the genomic instability of PTEN in clinically insignificant and significant prostate cancer showed that PTEN deficiency was predictive of biochemical recurrence in patients with a Gleason score ≥7, with 80% recurrence in patients with PTEN deficiency versus 55% in those with intact PTEN.47 A further study showed that patients with high-risk localized prostate cancer with PTEN deficiency had a shorter time to biochemical relapse (19 months) compared with patients without PTEN deficiency (106 months).48 In addition, a study in patients with low- and intermediate-risk prostate cancer indicated that PTEN deletion can be used to identify subgroups at greater risk of biochemical recurrence.49 These studies provided an early signal that PTEN deficiency is potentially associated with more rapid progression of disease. In alignment with this, PTEN deficiency was reported to significantly increase the risk of distant metastasis (p<0.01).50
Time to Progression and Progression-free Survival
PTEN deficiency was associated with a shorter time to non-metastatic relapse in patients with nmHSPC (median: 1.7 years for PTEN deficiency versus 5 years for intact PTEN; p=0.01),51 and there was a statistically significantly shorter recurrence-free survival (RFS) with homogenous deficiency versus intact PTEN in patients with nmHSPC (p=0.00152 and p=0.004453). Furthermore, a greater risk of progression after treatment with adjuvant docetaxel was reported in patients with nmHSPC (PFS at 18 months: 45.5% with intact PTEN versus 25.7% with PTEN deficiency).54 Shorter PFS has also been reported in patients with mHSPC and PTEN alterations (hazard ratio [HR]: 1.51; p=0.03).55
Overall Survival
PTEN loss or deficiency is associated with shorter OS in prostate cancer than intact PTEN. Foxley described the “quite staggering” separation in the Kaplan-Meier curves for OS in patients with high-volume de novo mHSPC and PTEN deficiency versus intact PTEN (p<0.001) and highlighted the “clear unmet need, considering the drop off in OS with PTEN deficiency” (Figure 2A).56 Patients with mHSPC and/or mCRPC and tumor PTEN deficiency were also shown to have worse OS than those with PTEN-positive disease (adjusted HR: 1.75; 95% CI: 1.19–2.55; p=0.004; Figure 2B).14 Recent data from the PROMISE Registry support that PTEN deficiency is correlated with poor OS in patients with de novo metastatic prostate cancer (HR: 0.73; p=0.003; Figure 2C).57
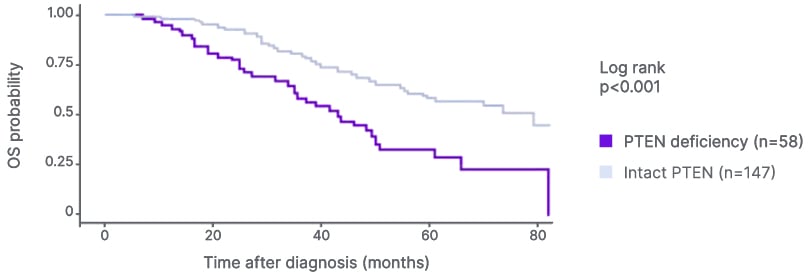
Figure 2A: Overall survival in patients with PTEN-deficient, high-volume de novo metastatic hormone-sensitive prostate cancer.56
Adapted from Zhang et al.56 2022.
OS: overall survival.
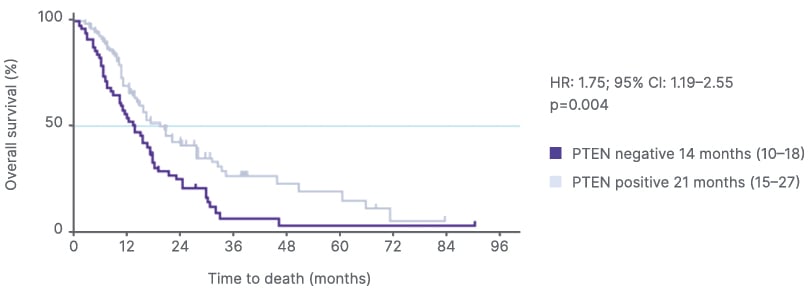
Figure 2B: Overall survival in patients with PTEN-deficient metastatic castration-resistant prostate cancer post-abiraterone.14
Adapted from Ferraldeschi et al.14 2015.
HR: hazard ratio.
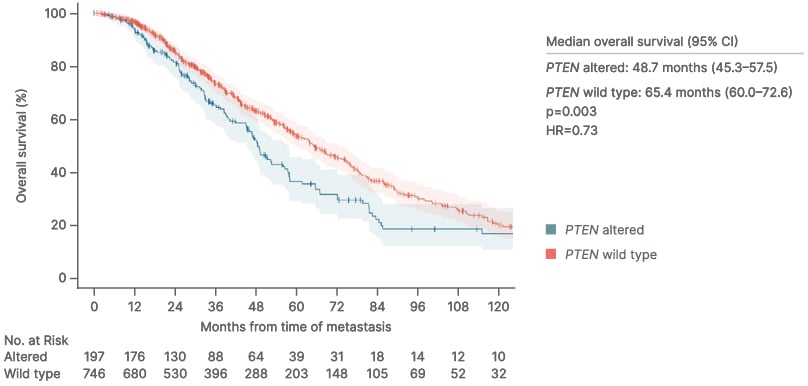
Figure 2C: PTEN deficiency and overall survival in patients with de novo metastatic prostate cancer (PROMISE registry).57
HR: hazard ratio
PTEN Deficiency as a Biomarker for Prostate Cancer
PTEN loss or deficiency occurs in approximately 25% of patients with de novo mHSPC and is associated with poor outcomes.14-17 Diagnostic technologies to test for PTEN deficiency and PTEN alterations in metastatic prostate cancer include immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), and next generation sequencing (NGS).
Genomic testing has various advantages: multiple mutation targets can be analyzed simultaneously using NGS,58 and heterozygous and homozygous PTEN alterations can be detected with high sensitivity using FISH.16 However, genomic analysis of PTEN is challenging in terms of defining the type of mutation or alteration to look for in the tumor to indicate that the PTEN is dysregulated. This is because not all genetic rearrangements or missense mutations will result in deficiency of the PTEN protein and/or confer inactivation of the PTEN pathway. Furthermore, NGS and FISH may underestimate the frequency of PTEN deficiency.59 Sample size and quality, cost, and high PTEN testing failure rates are also potential issues.16,53,60
IHC detects PTEN loss that is not caused by genetic alteration (e.g., in cases of PTEN loss of heterozygosity), which may not be detected by FISH.13 In addition, IHC requires less biopsy sample and is less prone to failure than NGS, and therefore has a higher test success rate in cases where tissue quality or volume is challenging.16 There is good concordance between IHC and NGS (85.5%)61 as well as between IHC and FISH (66–88%)53,62 for assessing PTEN status. NGS technical failure rates can be >30% for samples collected retrospectively, whereas IHC failure rates are <5%.60,63
In a real-world situation, Foxley explained, relying solely on NGS testing may result in missing a proportion of patients with PTEN-deficient disease, underscoring the importance of adding IHC to testing schedules to identify patients with PTEN-deficient prostate cancer. NGS is a useful testing option, but IHC is a potentially ideal method to identify PTEN deficiency more accurately.
The extent of PTEN deficiency might determine the sensitivity of targeted treatment. In the IPAtential150 trial of the AKT inhibitor, ipatasertib, in patients with mCRPC, varying the cut-off for the proportion of cells with PTEN deficiency indicated a differential treatment effect.61 In post hoc exploratory analyses, consistently better radiographic PFS benefit was observed when more stringent IHC cut-offs were used.61
Improvement in Radiographic Progression-free Survival with Capivasertib in the Phase III CAPItello-281 Study
Capivasertib, an AKT inhibitor,45 in combination with abiraterone, an anti-androgen, plus prednisone/prednisolone and ADT showed a statistically significant and clinically meaningful improvement in radiographic PFS (the primary endpoint) versus placebo with abiraterone plus prednisone/prednisolone and ADT in patients with PTEN-deficient de novo mHSPC in the Phase III study, CAPItello-281.42,64,65 Although the OS data were immature at the time of the analysis, there was an early trend towards an OS improvement in the capivasertib arm compared with the placebo arm. The safety profile of capivasertib in combination with abiraterone and ADT in CAPItello-281 was broadly consistent with the known profile of each treatment. The trial is ongoing and more mature data for key secondary endpoints, including OS, are awaited with interest by the medical community.
Conclusion
Despite the advances in therapy for mHSPC, disease progression to a castrate-resistant state (mCRPC) still occurs and is more rapid in PTEN-deficient disease, and survival rates for metastatic prostate cancer remain poor. PTEN deficiency is associated with poorer prognosis compared with intact PTEN function, as shown by shorter PFS, time to progression, and OS. Targeting both the AR and PI3K/AKT pathways simultaneously is important to drive down the reliance on the AR axis whilst the tumor is still reliant on androgen drive, and to drive down the reliance on the PI3K/AKT/PTEN pathway, which is amplified in PTEN-deficient disease. This “two brakes on the engine” approach might address multiple mechanisms of tumor growth and resistance. NGS testing alone is unlikely to detect all patients with PTEN-deficient tumors, underscoring the importance of adding IHC to testing schedules to identify patients with PTEN-deficient prostate cancer. The results with capivasertib in the CAPItello-281 study show that adding an AKT inhibitor to standard-of-care therapy provides benefit to patients with PTEN-deficient mHSPC, and indicates the potential role of this combination in an area of considerable unmet need.
Key Points Raised in the Question and Answer Session
One attendee inquired about data on PTEN expression in patients with high-risk prostate cancer at the initial risk stratification stage. Foxley explained that the medical community expected to see a correlation between PTEN deficiency and high-risk, high-volume disease, with the PTEN-deficient state driving disease progression harder, leading to bulky disease. However, data from the PROMISE Registry indicated that the negative effect of PTEN deficiency is more evident in low-risk, low-volume disease than in high-risk, high-volume disease, with the drop off in OS more dramatic in the former.57 Considering these data, assumptions cannot be made about the impact of PTEN deficiency on high-risk versus low-risk or high-volume versus low-volume disease, and drivers of disease rather than the extent of disease must be considered. The results also highlight that PTEN deficiency is an important factor in patients with low-risk, low-volume prostate cancer and testing for PTEN is needed in clinical practice. Foxley remarked: “PTEN deficiency within low-risk disease is potentially a ‘silent factor’ that would go unnoticed in the absence of testing.”
Another attendee asked about the extent of any overlap between PTEN deficiency and DNA repair mutations such as BRCA mutations. According to Zhengtao Qin, US Medical Lead, Prostate Cancer, AstraZeneca, Seattle, WA, USA, research indicates that there is an approximately 5% overlap between PTEN deficiency and homologous recombination repair mutations in mHSPC.66-68







