Interview Summary
The landscape of neurology biomarkers has evolved significantly in recent years, affording new clinical insights that are helping to reshape patient care. During an expert interview conducted by the European Medical Journal (EMJ), Simon Thebault, Assistant Professor at Montreal Neurological Institute, and attending physician at McGill University Health Centre, Canada, explored important advancements in blood-based neurology biomarkers, focusing on the key role of neurofilament light chain (NfL). NfL is a neurone-specific protein and biomarker of axonal damage that can provide novel insights into disease activity in multiple sclerosis (MS) and aid in prognostication. New innovations in blood-based testing for NfL are helping to facilitate the clinical application of this key biomarker to improve MS disease management.THE EVOLVING BIOMARKER LANDSCAPE IN NEUROLOGY
Biomarkers are measurable indicators of an underlying biological process that can aid in disease diagnosis and act as markers of disease progression, prognosis, and treatment response.1,2 “A biomarker is a surrogate,” Thebault explained.
“In a disease context, biomarkers are anything that can meaningfully tell us about the clinical endpoint of interest.”
“One thing we struggle with in neurology is many of our clinical diagnostic tests are quite insensitive and often confounded by other physiologic variables,” Thebault continued. “The advent of biomarkers enables us to more objectively and longitudinally monitor therapeutic responses.”
Thebault described the development of ultra-sensitive biomarker detection technologies as a breakthrough innovation that has enabled the nascent potential of an array of protein biomarkers in blood, such as NfL, glial fibrillary acidic protein (GFAP), phosphorylated tau (pTau), and apolipoprotein-E (ApoE), many of which were discovered decades ago, to finally be realised.3 “We can now measure concentrations in the blood, which has enabled us to increase our understanding of the pathology of various conditions and apply specific testing in clinical contexts,” he explained.
CLINICAL APPLICATIONS OF BLOOD-BASED BIOMARKERS
As Thebault outlined, blood-based biomarkers already play an important role in the clinical management of numerous neurological conditions.3,4 “Various tau isoforms and ApoE4 are useful in diagnosis and, potentially, even in a predictive susceptibility context for dementia,” Thebault noted. He also highlighted how biomarkers are “imperative” to accompany therapeutic advances for conditions such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS), “both to select patients for treatment and then prognosticate and monitor over the course of the disease”. In patients with traumatic brain injury “blood biomarkers can be useful in a prognostic context to stratify patients according to the severity of their concussion,” Thebault added.
In MS specifically, Thebault described how “NfL is leading the way as an early prognosticator of emerging disease activity both over the short and long term.”5,6 “In one cohort, we measured NfL in patients followed for more than 15 years, and a significant portion of the ultimate disabilities patients incurred was predicted by a baseline one-off NfL measurement,” he remarked.5
Thebault explained that NfL can provide important information to aid clinicians in guiding patient management. “We know MS is very heterogeneous; some patients have very aggressive disease and others have more mild disease. […] If we can capture the trajectory of a patient early on, that can be prognostically useful […] and, for the neurologist […] this is something we can use to inform our treatment decision-making.”
In testament to its biomarker utility as a measure of treatment response, Thebault highlighted how NfL is also assuming increasing importance as an outcome measure in MS clinical trials.2
NEUROFILAMENT LIGHT CHAIN: AN IMPORTANT MARKER OF NEUROAXONAL INJURY
“NfL is the most abundant of the neurofilament proteins in the axon. Processes that cause injury to those axons are reflected in elevated levels ultimately measured in the blood,” Thebault explained.7
“One of the miraculous things about NfL is how long it seems to stick around,” he highlighted, “giving us this window of opportunity to capture the new axonal damage as it’s occurring, and, therefore, meaningfully prognosticate.” He also described NfL as “a particularly stable protein, which makes the sample itself easy to handle and store.”
However, Thebault emphasised that, like all biomarkers, NfL can be affected by confounding factors such as age, BMI and comorbidities which “either need to be adjusted for or at least taken into consideration.”8
IMMUNOASSAYS FOR DETECTING AND MEASURING NEUROFILAMENT LIGHT CHAIN
Considering the clinical application of NfL in neurology settings, Thebault reiterated that “the transformational change can be precisely associated with the development of high-sensitivity biomarker detection technologies.”
He explained that “the standard ways of measuring NfL have generally focused on antibody detection techniques associated with a (fluorescent) colour change.” Several different immunoassays for the quantification of NfL have now been developed; however, Thebault cautioned that “a key point to note with any of these detection techniques is they’re highly dependent on the antibody that’s used, and different assays can have entirely different sensitivities.”9 The Atellica IM NfL assay developed by Siemens Healthineers (Tarrytown, NY, USA) uses Quanterix’s (Billerica, MA, USA) proprietary NfL antibodies, which Thebault described as “the early leader in the field.”9 In conjunction with clinical, imaging, and laboratory findings, the Atellica IM NfL assay is CE-marked (CE 0197) for the intended use as an aid in identifying adult patients between 18–55 years of age with relapsing MS (RMS) who are at a higher, versus lower, risk of MS disease activity, as defined by new or enlarging T2 MRI lesions within a 2-year period.10
For clinical application of NfL immunoassays in MS management, Thebault stressed that it is “important to demonstrate that the numbers are as reliable and objective as possible.” The prognostic performance of the biomarker test from Siemens Healthineers was evaluated using 570 samples from subjects with RMS collected as a part of a prospective clinical trial (NCT02792231).11 Results showed that a clinical cut-off level of 12.9 pg/mL was able to distinguish those patients at risk of radiological MS disease activity, with patients above this NfL threshold developing ~75% newer/enlarging T2 lesions per year on average (Table 1).10
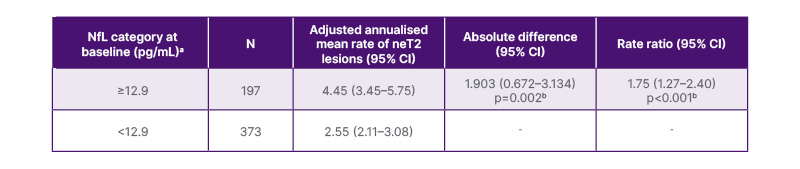
Table 1: Clinical performance of the Atellica IM neurofilament light chain assay (Siemens Healthineers, Tarrytown, NY, USA).10
aThe number of neT2 lesions (relative to baseline) was analysed in a negative binomial model with adjustments for NfL category and baseline T2 lesion volume. The natural log of the time from the screening scan (in years) was used as the offset.
bThe p value indicates the statistical significance (two-sided) at the 0.05 level.
neT2: new or enlarging T2 lesions; NfL: neurofilament light chain.
In terms of comparison/correlation across the different assays, Thebault highlighted choice of antibody as a key analytical test variable: “At least if the antibody is targeting the same epitope, as with the Quanterix antibodies, you could expect that the linearity of a test is preserved.” This expectation was observed in a recent head-to-head comparative study of the available automated NfL immunoassays in which the Atellica assay showed only small proportional bias against the SIMOA assay (Quanterix). Overall, this study showed a strong correlation between the four different assays evaluated, with an R2 value of ≥0.95.9
To facilitate application of NfL testing in everyday clinical practice, Thebault underscored the need for physician education “about specific assays and how to interpret these test results given the potential confounders.”
THE KEY ROLE OF NEUROFILAMENT LIGHT CHAIN IN CLINICAL MULTIPLE SCLEROSIS MANAGEMENT
According to recently published international consensus guidance, there is now substantial evidence to support the use of NfL, alongside MRI and clinical measures of disease progression, as a key decision-making tool for patients with MS.12 Current guidance therefore recommends regular NfL monitoring to determine change from baseline and predict subclinical disease activity, relapse risk, and the development of new Gd+ lesions.12 NfL can be incorporated into clinical practice as an adjunctive patient management tool for enabling expedited reassessment of patients with high or rising levels.13 Baseline measurement can have prognostic utility in helping to select the level of treatment efficacy and subsequent measurements, in addition to MRI, can aid in prognosticating future disease activity. Elevated NfL may signal the need to assess the patient more closely, monitor more diligently, or change/escalate therapy.12
UNLOCKING INSIGHTS INTO RELAPSING MULTIPLE SCLEROSIS WITH NEUROFILAMENT LIGHT CHAIN
Looking specifically at how NfL is currently utilised in the management of patients with RMS, Thebault described it as “primarily a clinical readout of neuroaxonal injury in the context of focal relapse biology, and to a much lesser extent, progressive, non-relapsing biology; another driver of clinical worsening in MS.”12,14
“Really, what it’s prognostic of is relapse-associated injury in MS,” he elaborated. “Clinically evident relapses are just the tip of the iceberg; beneath the surface lies a much larger burden of subclinical activity, for instance, as we see on MRI scans. NfL serves as a surrogate marker for this hidden activity, where it has prognostic value. Detecting subclinical elevations in NfL can provide an early warning signal, enabling neurologists to intervene proactively, potentially altering the disease course and preventing the emergence of new relapse-related inflammation.”12,14
“NfL level is perhaps representative of whether a patient is on a shallow or a steeper trajectory of their disease, which again, is prognostic, this time of longer-term outcomes,” Thebault continued. “It therefore provides a window of opportunity in relapsing MS to select a higher efficacy therapy, to escalate treatment to prevent clinically damaging disease activity.”12,14
THE EVOLVING ROLE OF NEUROFILAMENT LIGHT CHAIN
Thebault envisaged that the role of NfL as a biomarker in MS settings would continue to evolve and expand, highlighting potential future use “as discriminator of relapse versus pseudo relapse in that hyper-acute context.” He also suggested NfL could be used in treatment de-escalation strategies, both to assess patients before a therapeutic stop and to serially monitor for relapse risk after drug cessation.
Compared to traditional methods for managing patients with MS, NfL testing benefits from being relatively non-invasive, inexpensive, and amenable to frequent repetition.11 Therefore, “NfL provides an opportunity to supplement periodic data from an MRI,” Thebault explained. “If we’re not checking the MRI at a frequent enough interval, then we’re missing data, […] because we know a lot of the disease is happening subclinically, under the surface.” Measuring NfL levels can also offer “additional reassurance to patients that all is stable in the intervals between MRI.”
CONCLUSION
“The clinical scores we currently have [in MS] often lag years behind the disease changes that drove them. So, to have a more contemporaneous measurement that shows us damage as it’s occurring; that’s the prognostic utility of NfL,” Thebault summarised.
Currently, “we’re at the very beginning of the blood-based protein biomarker journey in neurology,” Thebault observed. Looking ahead, he highlighted NfL as “a prototype in a bursting pipeline of candidate proteins,” with different biomarkers likely to be combined in the future as part of a panel or “suite” of tests.15,16
“The revolution is the incremental gain that biomarkers offer in terms of being able to more precisely phenotype patients,” Thebault concluded. “The ultimate vision is personalised care in neurology…the idea of more targeted therapies on the basis of measuring something specific about an individual patient’s biology.”
All trademarks are the property of their respective owners.
NfL is not for sale in the USA. It may not yet be commercially available in all countries. Future availability cannot be guaranteed and is subject to local requirements.







