BACKGROUND
Alcohol-associated hepatitis (AH) is the most severe form of alcohol-related liver disease (ALD), which is characterised by an impairment of liver function associated with marked inflammation and poor prognosis.1 Therapeutic options remain limited, largely due to an incomplete understanding of the cellular and molecular mechanisms driving disease progression. Using single-cell RNA sequencing (scRNA-seq) of liver biopsies, the authors previously identified distinct hepatocyte and neutrophil subpopulations that were significantly enriched in AH.
AIM
In this study, the authors investigated the interplay between hepatocyte and neutrophil subpopulations enriched in AH, with particular emphasis on the serum amyloid A-formyl peptide receptor 2 (SAA-FPR2) signalling axis, to better understand their role in disease pathogenesis.
METHODS
The authors performed scRNA-seq on liver biopsies from patients with ALD encompassing disease spectrum (healthy controls: n=3; early ALD: n=3; AH: n=6). Distinct cellular subpopulations were characterised and validated via immunofluorescence (IF) in liver sections of patients with ALD. Cell–cell communication networks were analysed using a CellChat algorithm. Protein expression of hepatic SAA2 across ALD stages was evaluated by IF. Additionally, the authors developed a patient-derived liver organoid model treated with an ‘AH medium’, which contained pro-inflammatory stimuli (IL-1β, TNF-α, LPS, ethanol) to mimic the AH microenvironment. Gene and protein expression of acute-phase SAAs (A-SAAs), including SAA1 and SAA2, was evaluated in liver organoids by RNA sequencing and IF, respectively, while A-SAAs secretion levels were quantified using an ELISA test. Finally, plasma A-SAAs levels were measured in a well-characterised ALD cohort (healthy controls: n=9; early ALD [Stages F0/F1, stage F2, compensated cirrhosis]: n=50; decompensated cirrhosis: n=21; AH: n=60), and associations with clinical data such as disease stage, survival (at 1, 3, and 12 months), and bacterial infections were examined.
RESULTS
A scRNA-seq analysis identified two hepatocyte subpopulations enriched in AH (AH- Hepatocyte [1] and AH-Hepatocyte [2]), characterised by a high gene expression of A-SAAs, as well as a neutrophil subpopulation (AH-Neutrophil) that overexpressed IL1R1, as well as FPR2, a known receptor for SAA. In silico analysis revealed a strong interaction between these hepatocyte and neutrophil subsets via the SAA-FPR2 axis. The authors confirmed elevated protein expression of A-SAAs in AH-Hepatocytes (Figure 1), and of IL1R1 and FPR2 in AH-Neutrophils in liver sections of patients with AH. In hepatic organoids, A-SAAs expression at mRNA levels, and secretion levels, was significantly increased upon AH-medium stimulation. Moreover, A-SAAs circulating levels differed significantly across ALD stages (healthy: 7,388 ng/mL; early ALD: 23,884 ng/mL; decompensated cirrhosis: 17,948 ng/mL; AH: 54,232 ng/mL; p=0.0338, Kruskal–Wallis test). Post hoc analysis revealed that this difference was primarily driven by a significant increase in A-SAAs levels in patients with AH compared to healthy controls (p=0.0468). Among patients with AH, higher A-SAAs levels were significantly associated with short term mortality at 1, 3, and 12 months (p=0.0103, 0.0266, and 0.0180, respectively, using Mann–Whitney tests). A-SAAs levels were also elevated in patients with active bacterial infections (78,799 versus 40,009 ng/mL; p=0.0665, Mann–Whitney test).
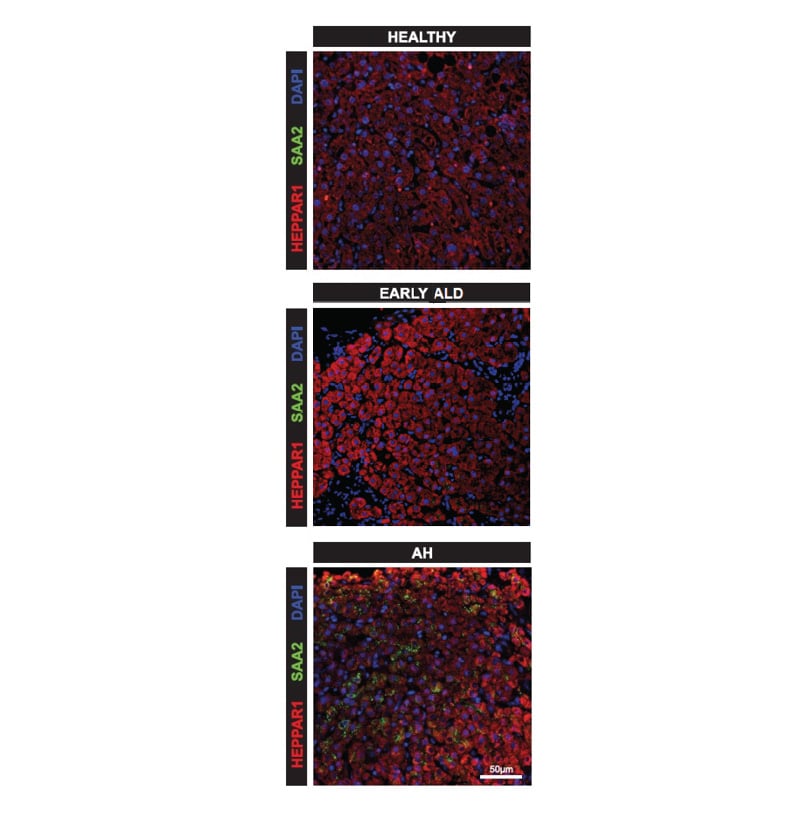
Figure 1: Elevated protein expression of SAA2 in alcohol-associated hepatitis-hepatocytes.
AH: alcohol-associated hepatitis; ALD: alcohol-related liver disease
CONCLUSION
This study highlights the pivotal role of SAA-FPR2 in mediating interactions between hepatocytes and neutrophils, two of the most relevant cell populations in AH pathogenesis. The authors show how A-SAAs increase their expression in response to inflammation, resulting in an intense interaction with neutrophils in the liver and A-SAAs secretion into the general circulation. This shows their potential as
a novel therapeutic target in AH.







