Abstract
Introduction: Neurocysticercosis, a parasitic infection caused by Taenia solium larvae, is a prevalent health challenge in regions with poor sanitation and among individuals migrating from endemic areas. This case report discusses the complexities of neurocysticercosis management.
Main symptoms and clinical findings: A 23-year-old male with neurocysticercosis-related hydrocephalus and paroxysmal sympathetic hyperactivity (PSH), presented with altered mental status, headaches, and neurological deterioration. MRI findings revealed numerous cystic lesions and characteristic findings indicative of neurocysticercosis, with subsequent CT imaging identifying progressive hydrocephalus.
Main diagnoses, therapeutic interventions, and outcomes: Neurocysticercosis with hydrocephalus and PSH was diagnosed. This necessitated the placement of a ventriculoperitoneal shunt and external ventricular drain. Despite initial improvement with antiparasitic and corticosteroid therapy, the patient’s clinical course was complicated by seizures and autonomic dysregulation. PSH was suspected due to episodes of tachycardia and diaphoresis, requiring intensified anticonvulsant therapy and sedative management with propofol. Treatment adjustments included dual antiparasitic therapy (albendazole and praziquantel) and rigorous intracranial pressure management.
Conclusion: This case highlights the complexities of neurocysticercosis management, particularly when complicated by hydrocephalus and PSH, emphasising the need for early recognition and dynamic treatment modifications. As neurocysticercosis increasingly affects non-endemic regions, healthcare systems must be equipped to recognise and treat its severe manifestations, with broader public health efforts focusing on prevention in endemic areas and among migrant populations.
Key Points
1. This case report explores the intensive care management of a young patient with neurocysticercosis, complicated by low-pressure hydrocephalus and paroxysmal sympathetic hyperactivity.2. The case highlights the delicate balance of cerebrospinal fluid diversion, timing of antiparasitic therapy, and autonomic stabilisation in a critically ill patient with central nervous system infection.
3. This emphasises the need for greater clinician awareness of low-pressure hydrocephalus and paroxysmal sympathetic hyperactivity, two under-recognised but serious complications that require timely, nuanced, and multidisciplinary care to improve outcomes.
INTRODUCTION
Neurocysticercosis, a parasitic brain infection caused by the larvae of Taenia solium that leads to cysts in the central nervous system, is a significant public health issue in regions with poor sanitation and in populations migrating from endemic areas. Humans become infected by ingesting T. solium eggs, which develop into oncospheres capable of migrating to various organs, with a predilection for the central nervous system.1 The clinical manifestations of neurocysticercosis vary widely depending on cyst location, burden, developmental stage (active, transitional, or calcified), and host immune response, encompassing a spectrum from asymptomatic infection to severe neurological complications.1,2 While seizures and epilepsy are the most common presentations, other manifestations, such as hydrocephalus and focal neurological deficits, further complicate timely diagnosis and management.1,3,4
A large-scale study identified generalised seizures as the most prevalent symptom (62.5%), followed by headache (37.5%), focal seizures (20.8%), and localising neurological signs (16.6%), with radiological findings most commonly showing parenchymal lesions (81%) and single lesions in over half of the cases (53.5%).5 Interestingly, dietary habits played a notable role, as 39% of patients were vegetarians, and only 17.6% reported pork consumption.5
Hydrocephalus is a well-recognised complication of neurocysticercosis, resulting from either direct obstruction by cysts or an inflammatory response causing cerebrospinal fluid (CSF) flow obstruction.5 It is present at the onset of the disease in 16–51% of patients.5 Notably, hydrocephalus associated with neurocysticercosis may exhibit distinct characteristics, such as low-pressure hydrocephalus (LPH), which poses challenges in both clinical and radiological diagnosis, due to its atypical pressure profiles and imaging findings.5,6 Current guidelines recommend surgical removal for hydrocephalus caused by viable cysts, while ventriculoperitoneal (VP) shunts and close monitoring for complications are better suited for degenerating cysts, which evoke widespread inflammation.5
Paroxysmal sympathetic hyperactivity (PSH) is a poorly understood condition characterised by episodic tachycardia, hypertension, hyperthermia, and diaphoresis following acute brain injury. PSH may exacerbate neurological deterioration and complicate ICU management if not recognised and addressed in a timely manner.7,8 This case illustrates the multifaceted challenges in diagnosing and managing the rare combination of neurocysticercosis with concurrent LPH and PSH.
CASE PRESENTATION
Patient InformationA 23-year-old Hispanic male, recently immigrated from Mexico, presented with altered mental status, headaches, nausea, and vomiting. His past medical, family, and psychosocial history were unremarkable, with no known genetic conditions or prior neurological issues. There was no family history of seizures, neurodegenerative diseases, or autoimmune conditions.
Clinical Findings
On admission, he exhibited confusion and intermittent agitation. Neurological examination revealed impaired cognition and bilateral upper extremity hyperreflexia. He had no prior history of neurological deficits or psychiatric illness.
Diagnostic Assessment Initial MRI revealed numerous round, symmetrically distributed lesions throughout both cerebral hemispheres, highly suggestive of neurocysticercosis ([/hl]Figure 1[/hl]). These lesions had a central area resembling CSF, surrounded by a sharply defined rim with adjacent white matter oedema. The characteristic ‘dual rim sign’ on post-contrast images, along with mild haemosiderin deposition in a lesion near the left lateral ventricle, supported the diagnosis. Additional filling defects and fluid levels in the lateral ventricles suggested cellular debris or cystic material, consistent with neurocysticercosis-related abscesses.
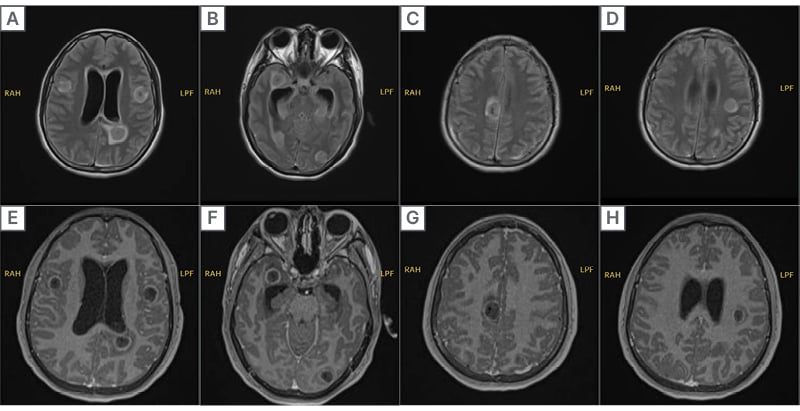
Figure 1: MRI of neurocysticercosis lesions.
Axial MRI images demonstrating multiple cystic lesions consistent with neurocysticercosis. The top row consists of T2-weighted and fluid-attenuated inversion recovery sequences, showing hyperintense cystic lesions with surrounding oedema. The bottom row displays T1-weighted post-contrast images, highlighting ring-enhancing lesions with perifocal oedema, indicative of an active neurocysticercosis infection.
CSF analysis showed a neutrophilic pleocytosis (white blood cells: 100 cells/µL; 87–98% neutrophils), elevated protein (up to 121 mg/dL), and normal-to-high glucose (47–79 mg/dL), with negative Gram stain and negative PCR for Herpesviruses and Toxoplasma gondii. HIV testing was non-reactive. T. solium IgG by ELISA was performed and the result was negative (4.0). This does not rule out neurocysticercosis, particularly in the setting of parenchymal or ventricular disease, where serological sensitivity may be limited.
Therapeutic Intervention
The patient was started on dexamethasone and albendazole as per infectious disease and neurosurgery recommendations. Due to hydrocephalus, a right parietal VP shunt with a low-pressure valve was placed (Figure 2). Shortly after, worsening symptoms and ventricular dilation necessitated revision. Albendazole was initiated only after the VP shunt and steroid therapy to prevent exacerbation of cerebral oedema. Continuous monitoring of beat-to-beat blood pressure and heart rate was performed using an arterial line, providing real-time, accurate measurements. The arterial line also facilitated serial sodium lab draws to maintain serum sodium between 140–150 mEq/L, in addition to routine labs.
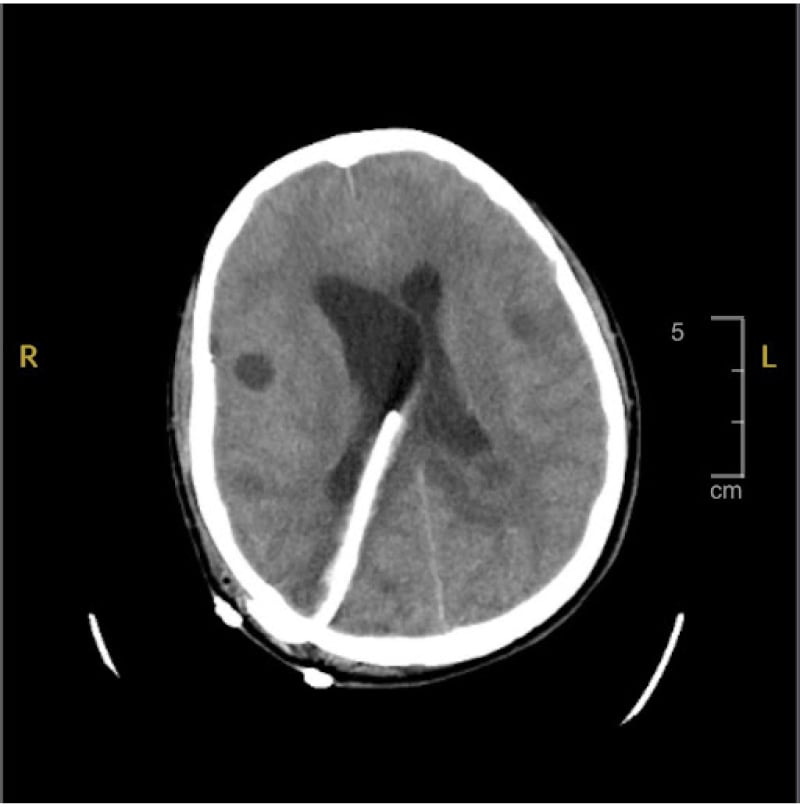
Figure 2: Head CT post ventriculoperitoneal shunt.
CT of the brain status post-right parietal approach ventriculoperitoneal shunt placement, with improved dilation of the ventricles compared to prior imaging.
His condition initially improved over the following three days, with increased alertness, spontaneous eye opening, and appropriate motor responses. He was subsequently downgraded from the ICU. However, on postoperative Day 7, he experienced an acute neurological deterioration marked by right-beating nystagmus and a rapid decline to a Glasgow Coma Scale score of 3. He was intubated for airway protection, and a CT scan revealed significant left lateral ventricle dilation, new left-to-right subfalcine herniation, and worsening hydrocephalus. Emergent left-sided external ventricular drain (EVD) placement was performed. Despite the presence of a functioning VP shunt, worsening hydrocephalus and herniation suggested shunt malfunction. A bedside shunt tap revealed minimal return and delayed refill, prompting urgent placement of an EVD for direct intracranial pressure (ICP) control.
Following intubation, the patient displayed seizure-like activity, including opsoclonus and head jerking, managed with IV lorazepam. PSH was suspected based on concurrent tachycardia (110–130 beats per minute) and diaphoresis. Levetiracetam 1,500 mg and lacosamide 100 mg were initiated twice daily. EEG findings showed generalised alpha activity with theta and delta slowing, consistent with dynamic encephalopathy and cortical irritability, but without seizure activity.
Throughout his hospitalisation, he remained critically ill with progressive cerebral oedema, likely due to the inflammatory response to dying parasites. Albendazole therapy was escalated with praziquantel due to clinical worsening. Sodium goals were adjusted to 145–155 mEq/L, managed with 3% sodium chloride boluses and sodium chloride tablets. His EVD was set at -5 mmHg for ICP management.
Follow-up and Outcomes
The most recent brain CT showed stable ventriculomegaly; unchanged, small-volume left-sided ventricular haemorrhage; and persistent multiple cerebral abscesses. The patient remained intubated and sedated on propofol, receiving aggressive antiparasitic, anticonvulsant, and supportive care. He had shown some neurological improvement, including withdrawal to pain, eye opening to voice, and ability to follow simple commands. Following extensive discussion with the ICU and neurosurgical teams regarding prognosis and goals of care, the patient’s family elected for transfer to Mexico for continued supportive treatment. He remained intubated at the time of transfer and subsequently passed away.
TIMELINE OF KEY CLINICAL EVENTS
Please refer to Table 1 for details on the timeline of clinical events from initial presentation to subsequent transfer to a facility in Mexico.
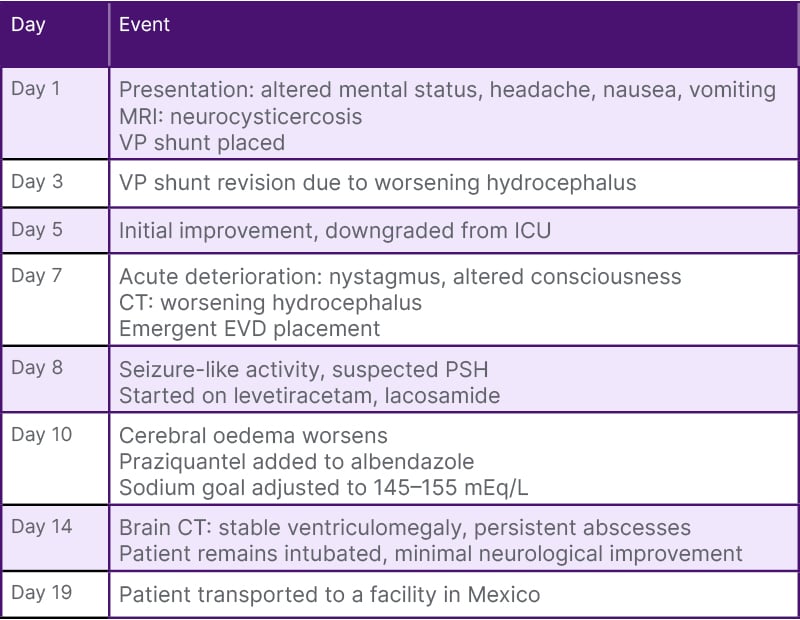
Table 1: Timeline of key clinical events.
EVD: external ventricular drain; VP: ventriculoperitoneal.
DISCUSSION
This case of neurocysticercosis, with the severe complications of LPH and PSH, highlights the immense clinical and diagnostic challenges inherent to managing this complex parasitic infection. Neurocysticercosis, caused by the larval form of T. solium, remains a significant burden in endemic areas and among migrant populations from these regions.2
Neurocysticercosis-Related Hydrocephalus
Hydrocephalus is a frequent and severe complication of neurocysticercosis, often resulting from cyst obstruction of CSF pathways or inflammatory reactions around cysts, leading to oedema and obstruction of CSF flow.1,3 Hydrocephalus in neurocysticercosis arises from mechanical obstruction and inflammation.5 Cysts obstruct CSF flow at key ventricular sites, causing acute symptoms like headache, or chronic issues such as gait abnormalities. Degenerating cysts trigger intense inflammation, leading to arachnoiditis, impaired CSF absorption, and fibrosis, compounding the obstruction and increasing morbidity.5
Neurocysticercosis-induced hydrocephalus can sometimes take the unusual form of LPH, where patients show clear signs of ventriculomegaly, but their CSF pressure readings appear deceptively normal, or even low.4-6 This can make diagnosis tricky, as LPH does not fit the typical pattern most clinicians expect in hydrocephalus cases.5 Because of this, recognising and treating LPH requires a different approach. As standard methods often fall short, innovative strategies, such as gently pressing on the valve intermittently to encourage drainage, or keeping the patient in a semi-reclined position, can make a big difference. In this case, the use of a low-pressure valve in the VP shunt and ensuring the patient remained in a semi-reclined position were integral to managing symptoms effectively and stabilising the patient’s condition. These methods have helped improve symptoms and lead to good recoveries, even when other treatments have failed.4,6 Acting quickly is key, as delays in addressing LPH can lead to long-term cognitive or neurological issues, making early recognition all the more critical.4,5
Paroxysmal Sympathetic Hyperactivity in Neurocysticercosis: A Compounding Factor
PSH, a condition characterised by episodic autonomic dysregulation (e.g., tachycardia, hypertension, and hyperthermia), is a recognised complication in patients with acute brain injuries, but is poorly understood in the context of neurocysticercosis and has been rarely reported in the literature.7,9,10 While PSH mechanisms remain under investigation, they are believed to involve disrupted autonomic regulation from hypothalamic or midbrain injury, potentially exacerbated by inflammatory processes.8,9
The suspicion of PSH led to modifications in the patient’s anticonvulsant regimen, with increased dosing of levetiracetam and lacosamide, and the initiation of sedative management using propofol. Although beta-blockers are a treatment option for managing symptomatic tachycardia and hypertension associated with PSH, they were not administered due to concerns about potential haemodynamic instability and the risk of exacerbating the patient’s bradycardia and hypotension. Instead, the focus was on optimising anticonvulsant therapy and sedation to control the PSH symptoms effectively, which allowed for better management of his complex condition.
Given that PSH can exacerbate neurological deterioration, rapid intervention was necessary to mitigate further brain injury and to stabilise autonomic fluctuations. This case reinforces the importance of early recognition of PSH symptoms, as failure to address them can lead to a cascade of detrimental effects, further compromising an already tenuous prognosis.10
Therapeutic Adjustments and Management Strategies
Initial antiparasitic therapy with albendazole was combined with corticosteroids to reduce inflammation secondary to parasite die-off, a critical step in minimising the inflammatory response associated with cyst lysis.3 Imaging studies, such as CT and MRI, provided detailed insights into the stage of the disease, revealing inflammatory changes around degenerating cysts and aiding in tailoring treatment.11 However, due to the patient’s continued clinical deterioration, praziquantel was added to the regimen, a decision supported by literature advocating dual therapy in severe or refractory cases.11 The significant cerebral oedema observed, likely triggered by an inflammatory response, necessitated intensive ICP management with hypertonic saline. Targeted sodium goals were employed, emphasising the importance of balancing effective ICP reduction with minimising risks such as hyperchloraemia and osmotic demyelination syndrome.12 These interventions highlight the necessity of combining neuroimaging advances, pharmacologic strategies, and ICP management protocols for optimising outcomes in severe neurocysticercosis cases.
This case serves as a stark reminder of the grim prognosis associated with advanced neurocysticercosis with concomitant hydrocephalus and PSH. Although the patient showed some responsiveness (e.g., withdrawal to pain and eye opening to voice), the high risk of permanent neurological impairment remained. In endemic areas and migrant populations, health education, improved sanitation, and screening for parasitic infections are vital to reducing the incidence of neurocysticercosis and its life-threatening complications.13 Physicians must recognise the importance of identifying neurocysticercosis as a global health issue, with implications for healthcare systems in non-endemic countries due to increasing migration and travel. The diverse presentation of neurocysticercosis, including LPH and PSH, demands that clinicians in non-endemic areas remain vigilant and well-informed to diagnose and manage this condition effectively.
CONCLUSION
Discussed herein is the rarity and clinical complexity of managing severe neurocysticercosis when complicated by LPH and PSH. LPH itself is an uncommon variant of hydrocephalus that defies the typical diagnostic patterns of elevated CSF pressure, making it especially challenging to identify and manage in regions where neurocysticercosis is rarely encountered. Similarly, PSH is seldom reported in the context of neurocysticercosis, adding another layer of diagnostic and therapeutic difficulty. These complications are more likely to be overlooked in non-endemic areas of the USA, where clinicians may have limited experience with the nuanced presentations of neurocysticercosis.
INFORMED CONSENT
The patient’s father (legal representative) provided informed consent for the publication of this case report, including the use of imaging and clinical details while ensuring confidentiality.







