INTRODUCTION
Pediatric patients who are tracheostomy dependent face an increased risk of respiratory infections due to bacterial colonization by Pseudomonas aeruginosa.1,2 Inhaled antibiotics, such as tobramycin, were developed as targeted treatments to reduce both the infection rates, and the need for systemic antibiotics. However, there is limited research on their use in children who are tracheostomy-dependent, particularly regarding their impact on healthcare utilization and optimal dosing.3,4
METHODS
The authors conducted a retrospective cohort study at Children’s National Hospital in Washington, D.C., including patients who are tracheostomy-dependent aged 0–21 years, from January 1, 2003–June 30, 2023. Patients with cystic fibrosis, bronchiectasis, or primary ciliary dyskinesia were excluded. The primary outcome measured emergency department visits, regular floor admissions, ICU admissions, and systemic antibiotic use 6 months before and after initiating inhaled tobramycin. The secondary outcome assessed changes in Pseudomonas aeruginosa positivity rates.
RESULTS
A total of 42 children were included, with a median age of 2 years; 64.3% were male. Neurologic disorders (45.2%) and bronchopulmonary dysplasia (26.2%) were the most common reasons for tracheostomy. Inhaled tobramycin therapy was associated with reduced regular admissions (p=0.04), ICU admissions (p=0.006), and systemic antibiotic use (p=0.05). Pseudomonas aeruginosa positivity decreased from 40.5% to 30.9% post-treatment (p=0.001; Table 1).
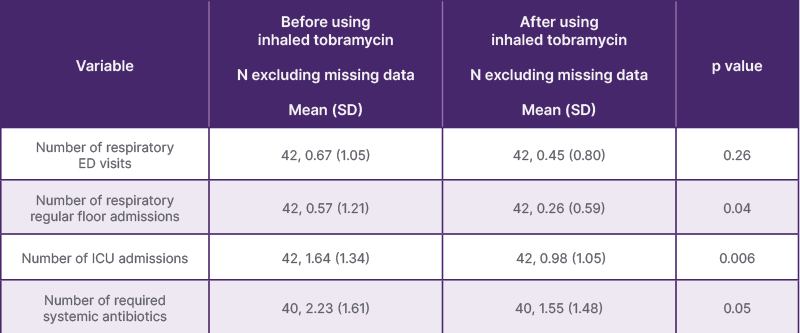
Table 1: Comparison of healthcare utilization before and after inhaled tobramycin therapy.
ED: emergency department.
CONCLUSION
This study, the largest involving children with tracheostomies who do not have cystic fibrosis, demonstrates that inhaled tobramycin significantly reduces hospitalizations, ICU admissions, and antibiotic use. Although 40% of patients received doses between 160–300 mg every other month, dose variability did not significantly impact clinical outcomes.







