Meeting Summary
Psoriasis is a common, chronic, inflammatory skin disease. The most common subtype, plaque psoriasis, typically presents as well-demarcated, erythematous plaques with scaling on the extensor surfaces, trunk, and/or scalp. Deucravacitinib is an approved oral medication for patients with moderate-to-severe plaque psoriasis who are candidates for systemic therapy in the USA, EU, and other countries.
This article summarizes findings from two clinical studies gathering additional data for the efficacy and safety of deucravacitinib in patients with moderate-to-severe psoriasis. In the 5-year POETYK PSO long-term extension (LTE) study, deucravacitinib had a consistent safety profile and sustained response. The PSORIATYK SCALP study included participants with more limited psoriasis than those included in POETYK PSO-LTE; compared with placebo, deucravacitinib was more effective at improving scalp-specific efficacy outcomes as early as Week 4, achieving clinically meaningful treat-to-target thresholds for overall body psoriasis, and improving quality of life (Dermatology Life Quality Index [DLQI]).
Deucravacitinib provides consistent efficacy and safety over 5 years in patients with moderate-to-severe plaque psoriasis, and represents a valuable treatment option in this population.
Introduction
Psoriasis is a common, chronic, inflammatory skin disease characterized by accelerated epidermal proliferation. The most common subtype is plaque psoriasis, which typically presents as well-demarcated, erythematous plaques with scaling on the extensor surfaces, trunk, and/or scalp.1 Estimates of the prevalence of psoriasis are limited and range from 0.5–11.4% of the population.2 In 2019, psoriasis affected 4.6 million people globally, including more than 324,000 in the USA.3
In psoriasis, dysregulation of the immune system in genetically susceptible individuals involves the IL-23-tyrosine kinase 2 (TYK2)-IL-17 axis.4
Management of psoriasis routinely involves drugs that target cytokines in the responsible inflammatory pathways.4 Mild psoriasis can be treated with topical corticosteroids, vitamin D analogues, calcineurin inhibitors, and emollients; however, moderate-to-severe disease may require phototherapy, oral drugs, or injectable biologics.1
Deucravacitinib is an oral, selective, allosteric TYK2 inhibitor that is approved in the USA, EU, and other countries for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy.5-9 TYK2 is an intracellular enzyme that mediates signaling of select inflammatory cytokines, including IL-23 and Type I interferons, which are involved in psoriasis pathogenesis.10,11 Deucravacitinib binds selectively to the TYK2 regulatory domain,10,12 representing the first in a new class of oral drugs. In the Phase III studies, POETYK PSO-1 and POETYK PSO-2, deucravacitinib 6 mg once daily (QD) was more efficacious than both placebo and apremilast at Week 16, and was well tolerated in this population.13,14
This article summarizes the latest data from two clinical studies that are gathering further data for the efficacy and safety of deucravacitinib: the POETYK PSO-LTE (NCT04036435) study in subjects with moderate-to-severe plaque psoriasis, and the PSORIATYK SCALP (NCT05478499) study in subjects specifically with moderate-to-severe scalp psoriasis.15,16
POETYK PSO-LTE
The main purpose of the ongoing Phase III POETYK PSO-LTE study is to characterize the long-term safety and efficacy of deucravacitinib (BMS-986165) in patients who participated in the POETYK PSO-1, PSO-2, and LTE trials.15
In the POETYK PSO-1 and PSO-2 trials, adults with moderate-to-severe plaque psoriasis were randomized 1:2:1 to oral placebo, deucravacitinib 6 mg QD, or apremilast 30 mg twice daily. Moderate-to-severe plaque psoriasis was defined as a Psoriasis Area and Severity Index (PASI) score ≥12, a static Physician Global Assessment (sPGA) score ≥3, and body surface area (BSA) involvement ≥10% at baseline. At Week 52, eligible participants could enroll in the POETYK PSO-LTE trial and receive open-label deucravacitinib 6 mg QD.17
Clinical efficacy of deucravacitinib in POETYK PSO-LTE has previously been well maintained through 4 years, with no new safety signals (compared to Year 3).11,18
Five-Year Efficacy and Safety17
Safety and efficacy of deucravacitinib were assessed up to the data cutoff of September 2nd, 2024, at Week 256 after the first treatment in the parent trials. The safety population included 1,519 participants who received ≥1 dose of deucravacitinib. Five-hundred-and-thirteen participants received continuous deucravacitinib and were included in the efficacy population.
At baseline, among the safety population, mean (SD) age was 46.6 (13.4) years, mean (SD) BMI was 30.5 (6.8) kg/m2, and 32.5% of participants were female. The majority (87.2%) of participants were White, and 10.1% were Asian. The mean (SD) disease duration was 18.7 (12.7) years, mean (SD) PASI was 21.1 (8.1), and mean (SD) BSA involvement was 26.2% (15.8%). A total of 1,211 (79.7%) of participants had an sPGA score of 3 (moderate), and the remaining 308 (20.3%) had an sPGA score of 4 (severe). Baseline data among the efficacy population were similar, with a mean (SD) DLQI score of 11.8 (6.1).
At data cutoff, the median duration of exposure among the safety population was 4.2 years (5,047 person years [PY]). Approximately 30% of participants had >5 years of exposure. The rate of serious infections was higher through 5 years than through 1 year; however, when COVID-19 was excluded from the analyses, the rate of serious infections was lower at 5 years (exposure-adjusted incidence rate [EAIR]/100 PY: 0.94 [95% CI: 0.69–1.25]) versus 1 year (EAIR/100 PY: 1.53 [95% CI: 0.86–2.52]). Incidence rates for adjudicated major adverse cardiovascular events (MACE) and malignancies were low and comparable through 1 year and 5 years. Incidence rates for malignancies decreased from 1 year through 5 years, with one new case of lymphoma reported from Year 4 to Year 5. No venous thromboembolic events were observed in Year 4 or Year 5. The EAIR/100 PY of other adverse events (AE) of interest related to the safety profile of deucravacitinib decreased in frequency from the 1-year to 5-year cumulative period, respectively, including acne (2.88 and 0.95), folliculitis (2.77 and 0.72), and oral ulcer (1.84 and 0.81).
EAIRs from the 5-year period remained consistent with findings from long-term clinical trial safety studies, disease registry, and real-world claims data of other approved psoriasis treatments for AEs of interest, including serious infections (excluding COVID-19), herpes zoster, MACE, venous thromboembolism, and malignancies.
A total of 207/513 (40.4%) participants in the efficacy population discontinued treatment before Week 256. Discontinuations were due to lack of efficacy in 16 (3.1%) participants, and AEs in 25 (4.9%) participants, with other common reasons being withdrawal by patients’, “other” miscellaneous causes as described by the participants, and study termination by the sponsor.
PASI 75, PASI 90, and sPGA clear to almost clear (0/1) response rates were sustained from Week 52 (beginning of the POETYK LTE trial) through 5 years (Figure 1). Efficacy results were consistent regardless of imputation method (observed values, treatment failure rules, and modified non-responder imputation).
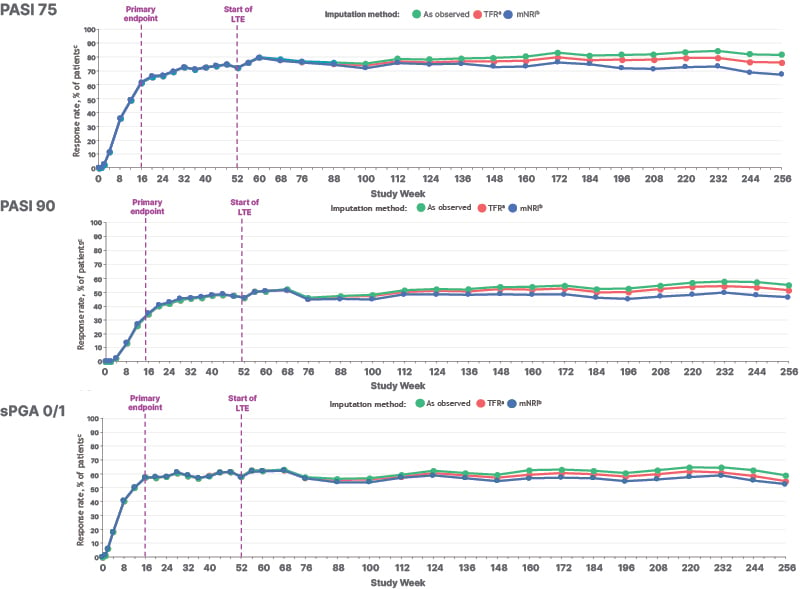
Figure 1: Response rates in the efficacy population of POETYK PSO-LTE over 5 years.17
ªTFR analysis captures discontinuations coded as “lack of efficacy.”
ᵇFor mNRI analyses, if there are no missing data (ie, no imputed values in the dataset), 95% CI was obtained using the Clopper-Pearson method based on the observed data.
ᶜData callouts represent the response rate (95% CI).
LTE: long-term extension; mNRI: modified non-responder imputation; PASI 90: ≥90% reduction from baseline in Psoriasis Area and Severity Index; sPGA: static Physician Global Assessment; TFR: treatment failure rules.
Five-Year Laboratory Data19
Further safety data from the 5-year analysis of POETYK PSO-LTE were presented at Diversity in Dermatology (DiD) 2025. Among the pooled POETYK PSO-1, PSO-2, and LTE safety population through to the cutoff date, the overall AE and serious AE rates decreased in frequency from the 1-year to 5-year cumulative period. After 5047 PY of deucravacitinib exposure, the AE rate was 127.4 EAIR/100 PY (95% CI: 120.61–134.48), and the serious AE rate was 5.06 EAIR/100 PY (95% CI: 4.44–5.75).
The most common AEs (EAIR/100 PY ≥5) were nasopharyngitis (9.12 EAIR/100 PY), upper respiratory tract infection (5.82 EAIR/100 PY), headache (2.62 EAIR/100 PY), diarrhea (2.12 EAIR/100 PY), arthralgia (2.65 EAIR/100 PY), and COVID-19 (8.22 EAIR/100 PY). Rates of serious infections (excluding COVID-19) were lower at 5 years than at 1 year, and malignancies and MACE were low and comparable through 1 year and 5 years.
A total of 106/1,519 (7.0%) participants in the safety population discontinued treatment due to AEs. Discontinuations due to laboratory abnormality AEs were rare, with 7/1,519 (0.005%) discontinuations over 5 years of deucravacitinib exposure. Three of these were due to a blood creatine phosphatase (CPK) increase, and one each were due to lymphopenia, hepatic function abnormality, alanine aminotransferase increase, and aspartate transferase increase. Overall, there were 11 deaths among the safety population, two of which were considered related to treatment by the reporting clinician: one patient with existing cardiovascular risk factors died due to aortic aneurysm rupture, and one patient died due to sudden death. Both deaths were considered unrelated to treatment by the investigator.
No clinically meaningful changes were observed through Week 256 in any of the evaluated laboratory parameters. These included chemistry parameters (alanine aminotransferase, creatinine, aspartate transferase, and CPK), lipid parameters (total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides), and hematology parameters (hemoglobin, neutrophils, lymphocytes, and platelets). This differs from changes in laboratory parameters, such as increased cholesterol, serum transaminases, and CPK levels, that are observed with Janus kinase inhibitors, another class of drug used to treat autoimmune diseases.20
PSORIATYK SCALP
Scalp psoriasis, which occurs in up to 80% of patients with psoriasis,21 is associated with pain, itching, bleeding, feelings of embarrassment, and a restriction in clothing choices,22 disproportionately impacting patients’ quality of life (QoL) compared with the severity of their disease as a whole.23 Scalp psoriasis can be challenging to treat with topical agents because hair makes the application difficult, and many patients find these treatments unacceptable.22
The Phase IIIb/IV, multicenter, randomized, double-blind, placebo-controlled PSORIATYK SCALP study aims to compare the efficacy and safety of deucravacitinib to placebo in participants with moderate-to-severe scalp psoriasis,16 including participants with less extensive overall psoriasis (BSA ≥3%) than those included in the POETYK PSO studies (BSA ≥10%).17,24
In PSORIATYK SCALP, adults were randomized 1:2 to placebo or deucravacitinib 6 mg QD. Moderate-to-severe scalp psoriasis was defined as having a scalp-specific PGA (ss-PGA) score ≥3, scalp surface area involvement ≥20%, and a Psoriasis Scalp Severity Index (PSSI) score ≥12.22 Baseline demographics and clinical characteristics were similar between the placebo (n=51) and deucravacitinib (n=103) groups.24
PSORIATYK SCALP achieved its primary and all key secondary endpoints, with a significantly greater proportion of participants treated with deucravacitinib achieving an ss-PGA score 0/1, ≥90% reduction from baseline in PSSI (PSSI 90), and a mean decrease from baseline in the scalp-specific numeric rating scale (ss-NRS) itch score at Week 16 compared with participants receiving placebo.25
Data were assessed for participants with baseline BSA 3–10% and BSA >10%; 108 participants were included in the BSA 3–10% group (70 in the deucravacitinib group and 38 in the placebo group), and 46 in the BSA >10% group (33 in the deucravacitinib group and 13 in the placebo group). At baseline, compared to the BSA 3–10% subgroup, the BSA >10% subgroup treated with deucravacitinib included fewer females (36.4% versus 47.0%), more participants with an ss-PGA score of 4 (39.4% versus 20.0%), and had a higher mean PASI score (17.4 versus 6.9).24
Time to Meaningful Improvement in Patient-Reported Outcomes24
Meaningful improvement in patient reported outcomes (PRO) was defined as a ≥4-point improvement in numeric rating scale (NRS) from baseline among participants with baseline NRS scores ≥4; ≥4-point improvement in DLQI from baseline among participants with baseline DLQI scores ≥4; and ≥20-point improvement in Scalpdex from baseline among participants with baseline Scalpdex scores of ≥20. It was evaluated using scalp-specific itch, pain, and flaking, and whole-body itch NRSs, Scalpdex, and DLQI.
Median time to meaningful improvement was quicker in participants receiving deucravacitinib versus placebo for each PRO evaluated (Figure 2). The median time to meaningful improvement in scalp psoriasis symptoms and whole-body itch ranged from 8–9 weeks for deucravacitinib; the median time was not reached for placebo. The median time to meaningful improvement in DLQI was 2.1 weeks for deucravacitinib and 11.7 weeks for placebo.
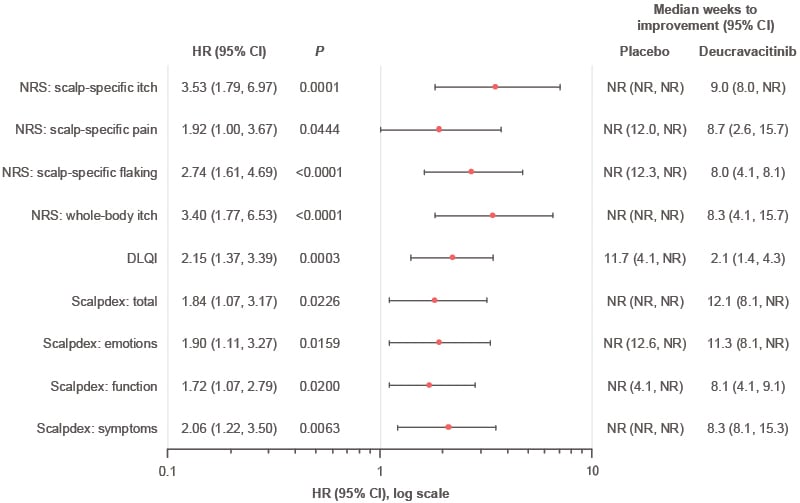
Figure 2: Hazard ratios associated with achieving meaningful improvement in patient-reported severity and quality of life measures by Week 16 in PSORIATYK SCALP.24
DLQI: Dermatology Life Quality Index; HR: hazard ratio; NR: not reached; NRS: numeric rating scale.
Greater and more rapid improvements with deucravacitinib versus placebo also occurred in participants with more limited overall psoriasis (BSA involvement ≥3%). Overall, time to meaningful improvement was shorter with deucravacitinib versus placebo for all PRO endpoints in the BSA 3–10% subgroup and the BSA >10% subgroup. However, these data should be interpreted with caution due the small sample size in the BSA >10% subgroup.
Treat-to-Target by Baseline Body Surface Area
While it improves patient outcomes and reduces costs in many other chronic diseases, the “treat-to-target” paradigm is not yet established in psoriasis.26 One post-hoc analysis of PSORIATYK SCALP data evaluated the ability of deucravacitinib treatment to achieve treat-to-target thresholds in subgroups of participants based on baseline BSA involvement.27
Absolute psoriasis area and severity index thresholds
Disease activity endpoints used in psoriasis clinical trials, such as PASI 75 and PASI 90, are limited in their applicability to clinical practice by their dependence on the accuracy of baseline PASI, and the potential for inter-assessor variability.26 In contrast, absolute PASI scores, such as 0/1 or ≤2 may represent more meaningful clinical and QoL-related endpoints to inform treat-to-target management strategies in the real-world.26 PASI ≤3 was also recently recommended as a treat-to-target strategy to define clinical remission in participants with moderate-to-severe plaque psoriasis.28
In this analysis of PSORIATYK SCALP, a greater proportion of participants receiving deucravacitinib achieved PASI thresholds of ≤1, ≤2, ≤3, ≤4, and ≤5 at Week 16 compared with placebo, both in the overall efficacy population (Figure 3) and subgroups with baseline BSA involvement of 3–10% and >10%.27
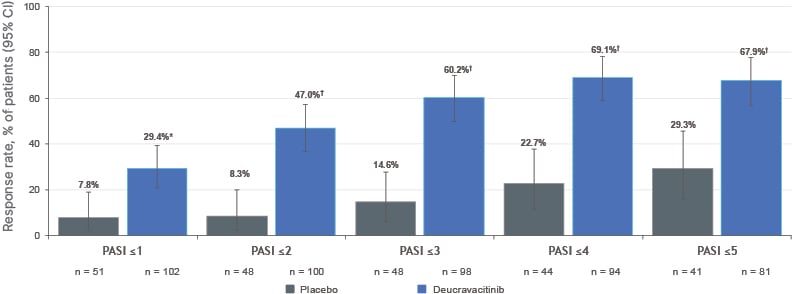
Figure 3: Proportion of participants achieving absolute Psoriasis Area and Severity Index threshold targets at Week 16 of PSORIATYK SCALP (non-responder imputation).27
*p=0.0023; ?p<0.0001 versus placebo. All p values are nominal.
Patients were included only if their baseline PASI was greater than the endpoint being evaluated.
PASI: Psoriasis Area and Severity Index.
Body surface area involvement thresholds
Alternatively, the consensus of the Medical Board of the U.S. National Psoriasis Foundation (NPF) is to use BSA involvement to set treatment targets. The NPF recommends a target response of BSA ≤1% at 3 months post treatment initiation. However, they recognize that, although BSA involvement thresholds are feasible in practice, they do not encompass QoL measures.29
In the PSORIATYK SCALP study, a greater proportion of participants receiving deucravacitinib versus placebo achieved various BSA thresholds at Week 16. A BSA threshold of ≤1% was achieved by 32/103 (31.1%) of participants treated with deucravacitinib and 4/51 (7.8%) of participants treated with placebo (p=0.0011), and a BSA threshold of ≤3% was achieved by 53/95 (55.8%) of participants treated with deucravacitinib and 5/44 (11.4%) of participants treated with placebo (p<0.001). Similar results were seen in subgroups with baseline BSA involvement of 3–10% and >10%.27
Improvement of the Dermatology Life Quality Index30
The DLQI is a skin disease-specific measure assessing six QoL domains: symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment. Scores range from 0–30, with higher scores indicating worse QoL.
In PSORIATYK SCALP, the DLQI questionnaire was completed at baseline and Weeks 1, 2, 4, 8, 12, and 16. In this post-hoc analysis, response rates for achieving a meaningful change in DLQI (≥4-point reduction in score from baseline) were assessed in participants with a baseline DLQI score ≥4 (placebo: n=47; deucravacitinib: n=95), and response rates for achieving a DLQI score 0–1 were assessed in participants with a baseline DLQI score ≥2 (placebo: n=50; deucravacitinib: n=99). Mean DLQI scores were similar between subgroups at baseline (9.5–12.1).
A greater DLQI change from baseline was reported from Week 1 with deucravacitinib compared with placebo. At Week 16, the adjusted mean (95% CI) change from baseline in DLQI was significantly greater in participants receiving deucravacitinib (-6.4 [-7.5– -5.3]) versus placebo (-1.5 [-3.0–0.0]; p<0.0001).
Greater proportions of participants receiving deucravacitinib reported no impact of psoriasis on QoL by Week 16, with higher DLQI 0/1 response rates observed from Week 8 compared with the placebo. Response rates for achieving DLQI 0/1 (95% CI) were greater in participants receiving deucravacitinib (32.3% [23.3–42.5]) versus placebo (10.0% [3.3–21.8]; p=0.0028), and response rates (95% CI) for achieving clinically meaningful improvement in DLQI were also greater in participants receiving deucravacitinib (69.5% [59.2–78.5]) versus placebo (29.8% [17.3–44.9]; p<0.0001).
Participants receiving deucravacitinib also experienced improvement from baseline in each DLQI subdomain, with improvements observed in participants with less extensive overall psoriasis (BSA 3–10%), as well as in those with BSA >10%. At Week 16, the adjusted mean (95% CI) change from baseline in DLQI was significantly (p<0.005) greater in participants receiving deucravacitinib versus placebo in each BSA subgroup.
Similarly, response rates for achieving clinically meaningful improvement in DLQI at Week 16 were significantly (p<0.005) greater in participants receiving deucravacitinib versus placebo in each BSA subgroup. DLQI 0–1 response rates were numerically greater in the BSA 3–10% subgroup and significantly greater in the BSA >10% subgroup.
Conclusion
In POETYK PSO-LTE, deucravacitinib had a consistent safety profile through 5 years with >5,000 PY of exposure. There was no increase in AE or serious AE rates over time, and no new safety signals emerged. PASI 75, PASI 90, and sPGA 0/1 responses were sustained through 5 years in participants treated continuously with deucravacitinib from Day 1 in the parent trials.17 No clinically meaningful changes were observed in any evaluated laboratory parameters, and discontinuations due to laboratory abnormalities were rare.19
Discontinuations due to laboratory abnormality AEs were low and balanced across treatment groups over the initial 52 weeks in parent trials.
PSORIATYK SCALP included participants with less extensive overall psoriasis and greater scalp involvement than the patient population in the POETYK PSO studies. Post-hoc analyses of PSORIATYK SCALP showed that deucravacitinib improved scalp-specific efficacy outcomes compared with placebo as early as Week 4, and these improvements were also observed in both BSA subgroups (3–10% and >10%).24 Deucravacitinib was also more efficacious than placebo in achieving a range of clinically meaningful treat-to-target thresholds for overall body psoriasis, and responses were consistent with those reported in the Phase III POETYK PSO-1 and PSO-2 trials, despite PSORIATYK SCALP including participants with less extensive overall psoriasis.27 In addition, participants receiving deucravacitinib reported greater improvements in DLQI overall, and in each subdomain, than those receiving placebo. Greater proportions of participants receiving deucravacitinib reported no impact of psoriasis on QoL by Week 16. These findings were similar in subgroups of participants with less extensive overall psoriasis (BSA 3–10%), as well as in those with BSA >10%.30
Deucravacitinib provides consistent efficacy and safety over 5 years in patients with moderate-to-severe plaque psoriasis, and is a valuable treatment option for patients.






