Summary
Hepatocellular carcinoma (HCC) is frequently diagnosed at an advanced stage, when most patients have underlying cirrhosis. Prognosis is influenced by both the tumor burden and the severity of chronic liver disease, and maintenance of liver function is crucial for optimizing the efficacy and safety of HCC therapy. This review explains the dual nature of advanced HCC and the need for clinicians to balance the management of liver function with treatment of the cancer itself. An overview of the current landscape of care and the interplay between liver function and HCC is provided, followed by the latest clinical trial data for the treatment of advanced HCC with regard to liver function. Real-world evidence for systemic therapies in patients with Child-Pugh (CP) B status is also described. Finally, key clinical implications for oncologists are presented, namely, that liver function influences prognosis, predicts treatment response, and impacts treatment selection, and that all patients with HCC require regular appointments with a hepatologist. This review provides clinicians with an understanding of the vital relevance of liver function in advanced HCC, supporting them to monitor and manage liver function to achieve the best possible outcomes for these patients.
INTRODUCTION
Liver and intrahepatic bile duct cancer are a substantial burden in the USA, with over 42,000 new cases predicted to occur in 2025.1 HCC represents the vast majority (~80%) of liver cancers.2,3 Most cases of HCC develop on a background of cirrhosis due to liver diseases such as chronic hepatitis B/C viral infection or non-alcoholic fatty liver disease (NAFLD).4,5
HCC carries a poor prognosis of 22% survival at 5 years, due to both frequent diagnosis at advanced stage and comorbid underlying cirrhosis, present in ~80% of those diagnosed.2,3 Approximately 40–50% of patients with HCC die due to complications of cirrhosis, rather than cancer progression.6 Since the prognosis of HCC relates to both tumor burden and the severity of chronic liver disease, HCC can be described as having a dual nature.3,7
In addition, maintaining adequate liver function is crucial to many of the treatment options available to patients with HCC.8 As liver function decline does not always coincide with disease progression, liver function needs to be monitored closely and adequately managed to sustain the therapeutic course.8 An improved understanding of the course of liver function decline in HCC is needed to help determine optimal treatment sequencing.8
This article aims to provide an overview of the literature regarding the dual nature of HCC and the need for clinicians to balance management of liver function with treatment of the cancer itself. For this narrative review, PubMed was searched for publications relating to both liver function and HCC over the past 10 years, and salient points were consolidated and summarized.
CURRENT LANDSCAPE OF CARE AND LIVER FUNCTION IN HEPATOCELLULAR CARCINOMA
Interplay Between Liver Function and Hepatocellular Carcinoma
In the early stages of cirrhosis caused by liver disease underlying HCC, the liver continues to function adequately despite the presence of scar tissue; this is termed compensated cirrhosis.9,10 Eventually, scarring reaches a level at which liver function becomes impaired, termed decompensated cirrhosis.9,10 At this stage, patients display clear symptoms such as jaundice, clinically significant portal hypertension (CSPH), ascites, variceal bleeding, and hepatic encephalopathy (e.g., confusion and sleep disorders).9,10 Patients with compensated cirrhosis who experience CSPH without variceal bleeding are considered to be at an increased risk of decompensation.11,12
Chronic liver disease and liver cirrhosis eventually cause severe impairment in liver function, which has a strong negative impact on prognosis in patients with HCC beyond that of the malignancy itself.10,13 Patients can also face complications such as portal hypertension and gut dysbiosis, and quality of life among patients with HCC significantly correlates with liver function.8,14 Life expectancy among patients with HCC with compensated cirrhosis generally exceeds 12 years; however, once liver disease has progressed to decompensated cirrhosis, survival expectancy drops to roughly 2 years.15
Assessing Liver Function in Patients with Hepatocellular Carcinoma
HCC staging is usually based on the Barcelona Clinic Liver Cancer (BCLC) system, which incorporates tumor size, number, and metastatic status with liver function and performance status.13 However, there are several techniques used to specifically assess liver function in patients with HCC.13
CP stage is based on ascites, hepatic encephalopathy, total bilirubin, albumin, and prothrombin time.16 CP stage is a grading system widely used to estimate the extent of liver resection that a patient can tolerate.16 However, its use to assess liver function in patients with HCC receiving systemic treatment has been criticized as insufficiently granular.10 The alternative system, albumin-bilirubin (ALBI) grade, is calculated based on serum bilirubin and albumin levels, avoiding subjective measures such as ascites and hepatic encephalopathy.16-18 The model of end-stage liver disease (MELD) scoring system is often used to determine a patient’s suitability for liver transplant but is less useful for assessing ongoing liver function in patients with HCC.13,16
Both CP stage and ALBI grade have a clinically prognostic role in patients with HCC who are receiving systemic therapy, and their use in clinical practice often depends on the experience and preferences of the individual center or clinician.13,19
Impact of Liver Function on Hepatocellular Carcinoma Treatment Options
Early-stage hepatocellular carcinoma
Treatment options for HCC include surgical, locoregional, and systemic therapies.4 For patients with early-stage HCC with CSPH, decompensated cirrhosis, and/or tumor multifocality, liver transplantation is considered the treatment of choice.4 Surgical resection is generally reserved for patients with localized disease in the absence of cirrhosis and/or without CSPH because of the risk of post-hepatectomy liver failure.4,13,20 It is far more common for patients with HCC to be treated with locoregional therapies such as ablation or transarterial therapy than resection in the USA.21
Local ablation therapy may be performed in patients with a single tumor who have CSPH.4 However, preserved liver function is still required because of the increased risk of post-interventional bleeding and deterioration of liver function in patients with decompensated cirrhosis.4,13 Since ablation is localized, the impact on liver function is limited, and decompensation following ablation therapy is rare.13
Patients with multinodular HCC may be treated with transarterial chemoembolization (TACE) or radioembolization (TARE) to slow tumor progression or downstage to other interventions.4,22 Patients with significant liver dysfunction, portal vein tumor thrombus, or a large tumor burden are generally considered to be unsuitable for transarterial therapy due to a higher risk of hepatic decompensation.4 Although transarterial therapies are effective treatments for HCC,23,24 they are associated with a deterioration in liver function.25,26 For example, TACE has been connected with a persistent, clinically meaningful deterioration in liver function-related biochemistry,25 and hepatic decompensation has been observed in 27.9% of patients with HCC treated with TARE.26 Radioembolization-induced liver disease, characterized by ascites and jaundice, has also been reported in a similar proportion of patients after TARE.27 These data suggest that patients treated with these approaches may eventually become unsuitable for locoregional therapy and require systemic therapy.25
Advanced-stage hepatocellular carcinoma
For patients who are not suitable for, or progress despite, locoregional therapy, systemic therapies can be offered, such as antiangiogenic targeted therapy or immune checkpoint inhibitors (ICI).4
In clinical trials, systemic therapies have primarily been evaluated in patients with preserved liver function (CP-A).23,28-32 However, in real-world clinical practice, patients with liver dysfunction, such as those with CP-B, are often treated with systemic therapy.33 In the absence of data from prospective clinical trials, treatment recommendations are predominantly based on evidence from retrospective, non-randomized studies and expert opinion, with an understanding that drug metabolism may be impaired in this population.33 There is some evidence to suggest that some systemic therapies may be well-tolerated in patients with CP-B cirrhosis, albeit with a possible decrease in efficacy,13 and therefore, some guidelines recommend these as options at first-line in patients with HCC and cirrhosis (with caution).20
The last decade has seen rapid developments in systemic therapies for advanced HCC,34 and a summary of some of the more recent data that relate to liver function is provided below.
SYSTEMIC THERAPIES AND THE CLINICAL IMPACT ON LIVER FUNCTION
Clinical Trial Data
Liver function-related data from several Phase III clinical trials of first-line systemic therapy in unresectable HCC (with CP-A) have been published recently, including the HIMALAYA,28 IMbrave150,29 REFLECT,31 and Checkmate-9DW trials.35 Table 1 summarizes data from clinical trials of systemic therapy in unresectable HCC with CP-A; it is important to note that data comparisons cannot be made between clinical trials, which differ in study design and geographical location.
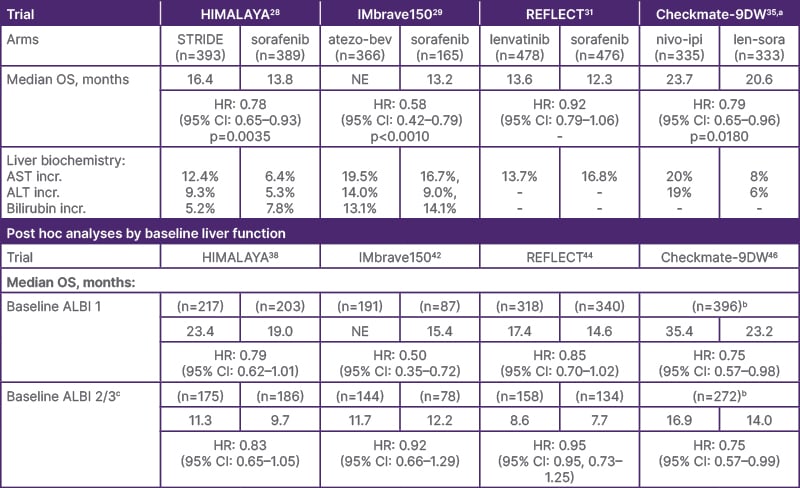
Table 1: Data from Phase III clinical trials of systemic therapy in unresectable hepatocellular carcinoma with CP-A.28-31
DISCLAIMER: Please note that this table is NOT intended as a comparison of data between clinical trials, which cannot be inferred in the absence of head-to-head studies.
aData displayed for this trial are from an interim analysis.
bData for individual study arms is not available.
ᶜThe REFLECT and HIMALAYA analyses did not include patients with a baseline ALBI of 3.
ALBI: albumin-bilirubin score; ALT: alanine aminotransferase; AST: aspartate aminotransferase; HR: hazard ratio; incr: increased; NE: not estimable; OS: overall survival; STRIDE: Single Tremelimumab Regular Interval (monthly) Durvalumab.
The HIMALAYA trial: tremelimumab plus durvalumab (STRIDE regimen)
The primary analysis of HIMALAYA showed that STRIDE (Single Tremelimumab, Regular Interval [monthly] Durvalumab) significantly improved overall survival (OS) versus sorafenib (Table 1).28 HIMALAYA also demonstrated durable long-term survival with an OS rate of 30.7% at 3 years, 25.2% at 4 years, and 19.6% at 5 years.28,36,37 Liver-related adverse events (AE) of increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were more frequently observed in the STRIDE arm, while serum bilirubin was more frequently observed in the sorafenib arm (Table 1).28
A post hoc analysis of 5-year data from HIMALAYA stratified patients by baseline ALBI grade.37,38 In patients with a baseline ALBI Grade of 1, the 5-year OS rate was 24.3% with STRIDE and 13.6% with sorafenib (hazard ratio [HR]: 0.79; 95% CI: 0.63–0.99).37 In patients with worse liver function (ALBI Grade 2/3), the 5-year OS rate was 13.7% with STRIDE and 4.7% with sorafenib (HR: 0.79; 95% CI: 0.63–1.00).37 The rate of treatment-related serious AEs with STRIDE was 20.4% in the ALBI Grade 1 group and 14.0% in the ALBI Grade 2/3 group.39 STRIDE is a preferred treatment option for patients with unresectable HCC,20 and study authors concluded that STRIDE was associated with long-term OS benefit versus sorafenib regardless of baseline liver function.38
The ongoing Phase III SIERRA trial40 is evaluating the STRIDE regimen in a broader population than HIMALAYA, including patients with CP-B.41 The co-primary endpoints of the trial are the incidence of Grade 3/4 AEs possibly related to study intervention and objective response rate (ORR), and interim data are anticipated in mid-2025.40
The IMbrave150 trial: atezolizumab plus bevacizumab
Atezolizumab plus bevacizumab (azeto-bev) is also a preferred treatment regimen for patients with unresectable HCC.20 The primary analysis of IMbrave150 demonstrated significant OS improvement with atezo-bev versus with sorafenib (Table 1).29 Increased AST and ALT were reported more frequently in the atezo-bev arm than the sorafenib arm, whereas increased serum bilirubin was reported in slightly more patients in the sorafenib arm.29
An exploratory analysis of IMbrave150 stratified patients by baseline ALBI score.42 In patients with ALBI Grade 1, OS improved with atezo-bev versus sorafenib (HR: 0.50; 95% CI: 0.35–0.72). In patients with ALBI Grade 2, no OS benefit was observed with atezo-bev versus sorafenib. The median time-to-deterioration in liver function (TTD; 0.5-point increase in ALBI from baseline) was 10.2 months (95% CI: 8.0–11.0) with atezo-bev versus 8.6 months (95% CI: 6.2–11.8) with sorafenib (HR: 0.82; 95% CI: 0.65–1.03). Study authors concluded that ALBI grade appeared to be prognostic for outcomes with ICI treatment, and that atezo-bev preserved liver function for numerically longer than sorafenib.42
A post hoc analysis of IMbrave150 assessed the incidence and prognostic role of hepatic decompensation following ICI therapy (either atezo-bev or sorafenib).43 Hepatic decompensation was defined as the occurrence of ascites, variceal bleeding, hepatic encephalopathy, or jaundice of Grade ≥2. The rate of decompensation over the first 3 months of treatment was 7%, compared with a rate of HCC radiological progression of 23%. Grade 2 bilirubin increase, international normalized ratio (INR) increase, and neoplastic macrovascular invasion were each independently associated with a higher risk of decompensation. Both early decompensation and early HCC radiologic progression were associated with higher mortality.43
The REFLECT trial: lenvatinib
Primary analysis of the open-label, non-inferiority REFLECT trial demonstrated non-inferiority in OS with lenvatinib compared with sorafenib (Table 1).31 Increased AST was reported in fewer patients treated with lenvatinib versus sorafenib.31
Post hoc analysis of REFLECT showed that median OS in the lenvatinib arm was higher among patients with a baseline ALBI Grade of 1 compared with those with a baseline ALBI Grade of 2 (17.4 and 8.6 months, respectively).44 However, the incidence of treatment-emergent AEs leading to discontinuation was higher in the ALBI Grade 2 group (13.3%) compared with the ALBI Grade 1 group (6.6%).
A retrospective analysis compared patients from REFLECT who deteriorated to CP-B within 8 weeks of randomization versus those who remained at CP-A.45 Patients with CP-B versus CP-A at Week 8 had an ORR with lenvatinib of 28.3% and 42.9%, respectively. Median OS was 4.5 months (95% CI: 2.9–6.1) in the CP-B group and 12.0 months (95% CI: 10.2–14.0) in the CP-A group. Grade ≥3 treatment-related AEs occurred at a rate of 3.7 /patient-year in the CP-B group versus 1.4 /patient-year in the CP-A group. The study authors concluded that continuation of lenvatinib may be a viable option in patients whose liver function deteriorates to CP-B during the first few weeks of therapy.
The Checkmate-9DW trial: nivolimumab plus ipilimumab
An interim analysis of the open-label Checkmate-9DW trial demonstrated significant OS benefit of nivolimumab plus ipilimumab (nivo-ipi;) versus lenvatinib/sorafenib (len-sora; 85% received lenvatinib) (Table 1).35 Regarding liver-related AEs in the nivo-ipi and len-sora arms, increased AST was reported in 20% and 8%, and increased ALT in 19% and 6% of patients, respectively.32 A total of 12 treatment-related deaths (4%) occurred in the nivo-ipi arm versus three (<1%) in the len-sora arm.
Post hoc analysis of Checkmate-9DW stratified patients by baseline ALBI score.46 In patients with ALBI grade 1, median OS was 35.4 months (95% CI: 23.9–not estimable [NE]) with nivo-ipi versus 23.2 months (95% CI: 21.4–28.3) with len-sora (HR 0.75; 95% CI 0.57–0.98).46 In patients with ALBI grade 2/3, median OS was 16.9 months (95% CI: 12.6–23.0) with nivo-ipi versus 14.0 months (95% CI: 11.1–16.6) with len-sora (HR 0.75; 95% CI 0.57–0.99).46 Safety across ALBI subgroups was generally consistent with the overall population.46 Study authors concluded that these data support the use of nivo-ipi as a potential treatment option for patients regardless of liver function.46
Together, these data indicate that newer systemic therapies for HCC are demonstrating improved outcomes for patients compared with sorafenib, though they have a variable impact on liver biochemistry. Post hoc analyses suggest that, although OS among patients with worse liver function at baseline (by ALBI score) may be shorter than that in patients with better liver function, the impact on liver biochemistry is similar for most (though not all) systemic therapies. These data should be considered with caution, as post hoc and exploratory analyses are not powered for statistical significance.
Similarly, a meta-analysis of studies investigating the efficacy or safety of ICI therapy for CP-B HCC found that although the safety of ICI treatment was comparable between patients with CP-A and CP-B, survival outcomes were inferior in those with worse liver function.47 Another meta-analysis, specific to the use of sorafenib at first-line, concluded that CP-B was associated with worse OS compared to CP-A, but that minor differences in response rate, safety, and tolerability are unlikely to be clinically meaningful.48
Robust data for the impact of systemic therapy on liver function are lacking, because liver decompensation has not been assessed as a clinical endpoint in most randomized controlled HCC trials.8 It has been suggested that measures such as time-to-decompensation and decompensation-free survival should be used in clinical trials for HCC to help delineate tumor progression from decompensated cirrhosis as a cause of poor outcomes.8 Head-to-head clinical trials comparing systemic therapies in patients with worse liver function are also needed to support treatment recommendations for this population.
Systemic Therapy: Real-World Data in Patients with Child-Pugh B
Although there is currently a lack of evidence from clinical trials to support the use of systemic HCC therapy in patients with impaired liver function, several real-world studies have included patients with CP-B, and a selection of these data is summarized below.
GIDEON, a prospective, observational registry study of sorafenib, included 1,968 patients with CP-A and 666 patients with CP-B.49 Median OS was longer in the CP-A group (13.6 months; 95% CI: 12.8–14.7) than in the CP-B group (5.2 months; 95% CI: 4.6–6.3). The incidence of drug-related events leading to discontinuation was similar between CP-A (17%) and CP-B (21%) groups, suggesting that the safety profile of sorafenib was consistent across patients with preserved and deteriorated liver function.
A retrospective cohort study of the STRIDE regimen included 87 patients with CP-A and 30 with CP-B.50,51 Median OS was NE (95% CI: 12.3–NE) in CP-A patients and 6.2 months (95% CI: 2.9–11.8) in CP-B patients. Grade 2–4 treatment-emergent AEs occurred in 42.5% of CP-A patients and 32% of CP-B patients, with immune-related AEs leading to discontinuation in two CP-B cases. Median time to 2-point worsening in CP was 11 months, and ALBI score was stable during treatment (among survivors). The authors concluded that STRIDE was tolerable in patients with CP-B in the study.
A retrospective study of atezo-bev included 133 patients with CP-A and 36 with CP-B.52 Median OS was not reached in the CP-A group and 7.7 months (95% CI: 4.8–10.6) in the CP-B group. ORR and disease control rate were 34.6% and 76.7%, respectively, in the CP-A group, and 11.1% and 58.3%, respectively, in the CP-B group. Grade 3–4 AEs were significantly more common in the CP-B group than the CP-A group (44.4% versus 15.8%; p<0.001), suggesting that, although modest clinical activity of atezo-bev was observed in patients with CP-B, careful evaluation of treatment response and AE management are required in this population.
Nivolumab was assessed in 132 patients with CP-A and 71 with CP-B in a retrospective, real-world study.53 Median OS was longer in patients with CP-A compared with those with CP-B (42.9 versus 11.3 weeks; HR 3.02 [95% CI: 2.15–4.24]; p<0.001). Treatment-related Grade ≥3 AEs led to treatment discontinuation in 3.8% of patients in the CP-A group and 1.4% in the CPA B group. The authors stressed the importance of careful selection of patients with CP-B for treatment with nivolumab, given an unsatisfactory response to treatment in this study population.
Together, these real-world data suggest that patients with CP-B may still be able to achieve responses with some systemic HCC therapies, albeit to a lesser degree than patients with CP-A. However, the toxicity of systemic therapies in patients with CP-B appears to vary, and this should be considered when selecting treatment for patients with moderate liver dysfunction. Randomized clinical trials are needed to understand the clinical relevance of these real-world data, and comparative studies will be important to determine the most suitable therapies for different patient subgroups.
CLINICAL IMPLICATIONS FOR ONCOLOGISTS
Liver Function Influences Prognosis10,13
The prognosis of patients with HCC is determined by both the cancer itself and the degree of liver function and cirrhosis.
Liver Function Predicts Treatment Response4,33,50
Deterioration of liver function can have a negative impact on the response to some HCC therapies.
Liver Function Impacts Treatment Selection10,13-15,52
Liver function should be considered when selecting the most suitable treatment for each patient. Patients with adequate liver function may achieve greater benefit from systemic therapies compared to patients with liver dysfunction. The individualized risks of treatment should be communicated to all patients.
All Patients with Hepatocellular Carcinoma Require Regular Liver Function Monitoring8
Regular appointments with a hepatologist should be maintained even if patients are receiving systemic therapy under the care of an oncologist.
CONCLUSION AND FUTURE DIRECTIONS
Improvements in systemic therapy for unresectable HCC over the past decade have vastly increased the choices available for treatment,34 with associated improvements in outcomes.23,28-32 However, because of the insidious nature of HCC and the underlying conditions associated with its development, a substantial proportion of patients with HCC present with liver dysfunction.2,3,7 Therefore, despite the focus of most clinical trials on patients with adequate liver function, patients with liver dysfunction are often treated with systemic therapies based on retrospective or real-world studies and clinician experience.33
Post hoc analyses of trial data have provided further evidence for oncologists to make treatment decisions for their patients with, or at risk of, liver dysfunction. Nevertheless, the design of clinical trials in HCC to include patients with CP-B, such as the ongoing SIERRA trial,40 is a positive development in the field. The addition of measures such as time-to-decompensation and decompensation-free survival to future trials would also be welcome.8
The dual nature of HCC, in which prognosis is based on both liver function and cancer progression, makes it critical for clinicians to balance the benefits of HCC therapy with the potential harm to liver function, and underlines the importance of multidisciplinary care in this patient population.13 Liver function assessments should guide the allocation of therapy, particularly in patients with advanced cirrhosis or compromised liver function, with the goal of improving outcomes for these patients.13







