PM-GBL-EXD-SMP-250001 | August 2025
Meeting Summary
At the European Academy of Allergy and Clinical Immunology (EAACI) Congress 2025 in Glasgow, UK, two experts in otorhinolaryngology and allergology joined the EAACI Patient Organisations Committee Chair to discuss the burden of disease in severe asthma and chronic rhinosinusitis with nasal polyps (CRSwNP). They also explored the potential of IL-5 inhibition as a therapeutic strategy, as investigated in the REALITI-A, SYNAPSE, and MESILICO studies. Additionally, experts discussed the concept of the unified airway, exploring how the upper and lower airways may function as an interconnected system and share common mechanisms.Living with CRSwNP: Navigating the Care Pathway
Özlem Ceylan, EAACI Patient Organisations Committee Chair, Istanbul, Türkiye, introduced CRSwNP as a debilitating, chronic inflammatory disease which affects 1–2% of the European population, amounting to around 8.9 million Europeans.1,2
Ceylan emphasised that the burden of disease is often overlooked, with patients facing substantial daily challenges impacting physical and mental health, social life, work, sleep, and healthcare resource utilisation, as well as incurring direct and indirect costs.3-5 She urged clinicians to look beyond the initial/immediate clinical status and focus on the whole patient journey, from before diagnosis to follow-up.
She shared results of an online survey carried out by the initiative BeyondTheNose, to explore the impact of CRSwNP on patients’ lives across Belgium, France, Germany, Italy, and Spain (N=168).5,6 The survey was designed to capture the burden of disease and the patient experience from diagnosis through to follow-up.6 Patients were included based on criteria of a medical diagnosis of CRSwNP and at least one of the following: experience of anosmia/hyposmia, currently taking a biologic agent, or having had one surgery to remove polyps. Of all respondents, 58% were male, and the majority (45%) were 46–50 years of age. In terms of treatment, 39% of patients had received courses of oral steroids, 30% were on maintenance oral steroids, 24% had received steroid injections, 37% were on biologic therapy, and 16% had undergone surgery. The most frequently reported comorbidities included allergic rhinitis, asthma, and food allergies (Figure 1).6
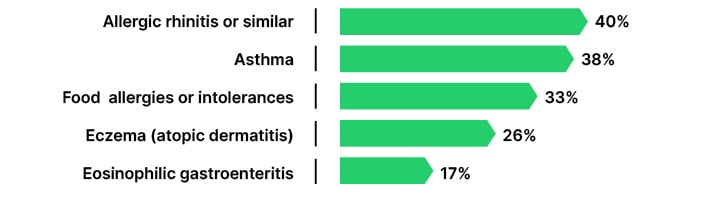
Figure 1: The top five comorbidities reported by patients with chronic rhinosinusitis with nasal polyps in the 2025 BeyondTheNose patient survey (N=168).6
Ceylan noted: “The impacts of CRSwNP extend far beyond the nose…they affect every corner of the patient’s life.” Poor sleep was reported in 77% of patients, leaving them feeling drained during the day, while 71% reported reduced physical activity and ability to participate in sports. Ceylan emphasised: “These insights are more than just numbers… they tell the story of daily challenges and gaps in care, and should guide us on how to improve the system altogether.”
Time to Diagnosis
All patients surveyed experienced delays in reaching CRSwNP diagnosis (97%), waiting an average of 4.2 years.6 Ceylan described the diagnostic journey for patients as “being lost in a foggy forest without a map, pointed in the wrong direction…as they tried to find their way, the signposts were confusing and kept leading to the completely wrong destination.” With current inefficient diagnostic pathways, there is an increased strain on healthcare costs and resources, worse symptoms, and higher frustration.6 She added that this highlights the need for a streamlined referral system and a proactive approach to diagnosis.
Access to Care
Although high levels of satisfaction were reported with the approach to the care provided by healthcare professionals (HCP), 96% wanted more support in the patient journey. Seventy-five percent of patients reported that it took several appointments to receive a diagnosis, and 47% needed more support accessing their prescribed treatment.6 Ceylan explained: “Access is not just about entry to the system, it’s about meaningful engagement and support once diagnosed, follow-ups, and treatment.”
Treatment Experience and Follow-up Care
Whilst the treatment experience is generally perceived well, treatment burden remains high. Patients reported taking an average of 5.5 different types of treatment, which Ceylan explained have varying degrees of success. In those prescribed short-term oral steroids (n=126), 65% had undergone two to three cycles in the past year. In patients who had surgery to remove polyps (n=100), there was a median of two surgeries in the past 3 years. Patients valued consistency in appointments and recognised the value of coordinated multidisciplinary follow-up care.6
Meeting Unmet Patient Needs in the Care Pathway
For a satisfying care experience, patients noted the value of knowing who to contact, reduced wait time for appointments, confidence to discuss the disease, understanding treatment options, defining treatment goals with HCPs, and having their treatment expectations met.6 A member of the audience asked Ceylan about the main unmet need in the patient journey. She emphasised that all the issues are equally important, but there is a pertinent need for early diagnosis, as everything starts from there.
Transforming Unified Airway Care with Emerging Science
Quirce highlighted a position paper published in 2020 by a panel of experts on the European Forum for Research and Education in Allergy and Airway Diseases (EUFOREA), which outlined key features defining remission in CRSwNP (Figure 2).2
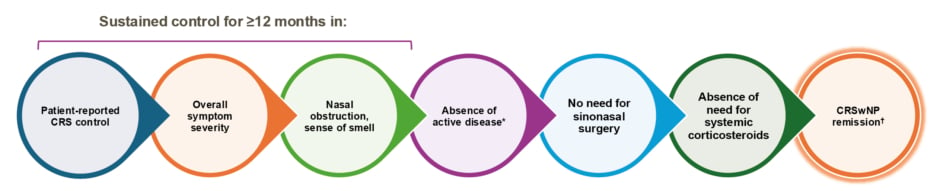
Figure 2: Key features for defining remission in chronic rhinosinusitis with nasal polyps.2
*Evaluated by nasal endoscopy.
✝ Remission can be reached without treatment, excluding systemic corticosteroids and surgery (in the last 12 months).
CRS: chronic rhinosinusitis; CRSwNP: chronic rhinosinusitis with nasal polyps.
As the treatment landscape evolves, remission in patients with CRSwNP and severe asthma is becoming an attainable goal.2,7,8 Quirce explained that emerging data shows patients with severe asthma can also aim for clinical remission, which means freedom from exacerbation for 12 months, freedom from oral corticosteroids (OCS) and/or systemic corticosteroids (SCS), symptom control, and stable lung function.7-9 In a recent systematic review and meta-analysis, Quirce stated that it was shown that 30% of patients with severe asthma treated with biologics achieve remission from the disease.10
The Core Role of IL-5 in Type 2 Inflammation
Quirce highlighted that recent research has described IL-5 as a pleiotropic cytokine, orchestrating a range of multi-directional effects across different structural and immune cells.11-18 IL-5 is involved in the differentiation, proliferation, survival, migration, and activation of eosinophils in blood and tissue,11,12 as well as enabling mast cell cross-talk.11,12 It also promotes basophil activation,12 expression and activation of IL-5 receptor alpha subunit (IL-5RA) on neutrophils, upregulates genes involved in plasma cell proliferation,12 downregulates genes relating to tight junctions and barrier function in ciliated epithelial cells, and modifies epithelial activation.12,15-17 IL-5 has also been shown to promote the proliferation and activation of fibroblasts,12 and mediation of smooth muscle cell activity,12,16 innate Type 2 lymphoid cells (ILC2),12 and T regulatory cell (Treg) production.12
In severe asthma, dysregulation of IL-5 drives Type 2 inflammation through effects on multiple cells, as characterised by elevated Type 2 biomarkers such as fractional exhaled nitric oxide (FeNO) and eosinophil count.15,16,19 Alarmins at the epithelial barrier release thymic stromal lymphoprotein (TSLP), IL-25, and IL-33, resulting in increased epithelial permeability. This leads to impaired pathophysiologic features and a dysregulated immune response. As a result, the clinical impact on patients includes symptoms such as persistent cough, wheezing, dyspnoea, exacerbations, and loss of lung function.19
IL-5 as a Therapeutic Target in Severe Asthma
Epithelial Barrier Dysfunction
Quirce overviewed a transcriptomic analysis of nasal brushes taken from patients with severe asthma at baseline and after 3 months of IL-5 inhibition (n=27), explaining that differential gene expression and DNA methylation analyses identified 6,719 genes and 53 CpG sites changed in response to IL-5 inhibition.20 Pathway analysis of gene expression changes showed evidence of suppression of inflammatory pathways and upregulation of genes relating to repair responses in epithelial cells, suggesting broad effects on the airway epithelium in severe asthma.20
He also introduced the MESILICO study, a multicentre study in Greece that measured epithelial damage through bronchial biopsies before and after 12 months of anti-IL-5 therapy in patients with severe asthma (N=30).18 IL-5 inhibition was shown to significantly reduce bronchial epithelial damage, a key feature of lower airway remodelling. Treatment was also associated with improved disease control and improved lung function versus baseline (before treatment initiation).18
Immune Imbalance and ILC2 Cells
A key component of the Type 2 inflammatory response in both asthma and CRSwNP is the involvement of ILC2 cells.21,22 IL-5 inhibition in patients with severe asthma has demonstrated the ability to rebalance T-effector cell and T-regulatory cell populations, as well as inflammatory cytokines.23
Mucus Plugging
By decreasing eosinophil and galectin-10 levels, IL-5 inhibition has also been shown to prevent the formation of mucus plugs.24-26 Ongoing studies aim to further investigate the role of IL-5 inhibition in lung function and ventilation associated with mucus plugs.27
Airway Remodelling
Quirce explained that often in patients with severe asthma, damage to the airway at the epithelial level is observed, with increased thickness in the sub-basement membrane and airway smooth muscle.18 In the MESILICO study, patients exhibited a significant decrease in measures of the airway wall, observed at 12 months post-anti-IL-5 treatment (p<0.01 and p=0.004).18 Overall, Quirce emphasised: “One of the main takeaways from this emerging evidence is that we can aim beyond clinical remission in severe asthma, that we can aim for disease modification.”24
Breathing as One: Integrating the Upper and Lower Airways
Quirce introduced the concept of a ‘unified airway’, that the upper and lower airways exhibit shared functional and histological characteristics.28 He emphasised that the common underlying mechanism seen in CRSwNP, severe asthma, and non-steroidal anti-inflammatory drug exacerbated respiratory disease (NSAID-ERD) is Type 2 inflammation driven by eosinophilic activity, and elevated levels of IL-5, IL-13, and IL-4.29
Quirce shared that a 2023 Spanish study in patients with Type 2 inflammatory disease (N=404) found that up to 70% of patients with CRSwNP have comorbid asthma, and 10% have comorbid NSAID-ERD. In patients with asthma, 40% have comorbid CRSwNP, and up to 15% have comorbid NSAID-ERD.29,30,31
Patient Case Study
Quirce introduced a hypothetical 54-year-old living with asthma since age 40 years. Over the past 5 years, her asthma has worsened, and she receives oral medication and a short-acting beta-2-agonist metered dose inhaler. Her symptoms include congestion, night awakenings, an increased use of her rescue inhaler, and an ongoing cough with thick off-white sputum.
She uses an inhaled corticosteroids (ICS)/ long-acting beta antagonist (LABA) inhaler, a long-acting muscarinic agonist (LAMA) inhaler, nasal steroid spray, and antihistamines. She suffers from comorbidities including CRSwNP, obstructive sleep apnoea, and hypertension. Reflecting on her case, Quirce commented: “In patients with CRSwNP and asthma, there is a link between the upper and lower airways. We need to address this connection and identify a common mechanism…this can help inform treatment, which can enable a bidirectional and simultaneous effect.”
However, Quirce remarked, it is not always easy to identify the phenotype or the endotype. The ideal course of action for diagnosis and predicting treatment response, as outlined in the Global Strategy for Asthma Management and Prevention (GINA) Guidelines 2025, is to measure Type 2 inflammation biomarkers. Blood eosinophil count (BEC) and FeNO can aid in diagnosing, phenotyping, monitoring of prognosis, and predicting treatment response in asthma.7 The GINA 2025 guidelines acknowledge the variability in BEC and FeNO, highlighting the importance of repeated testing, particularly in patients with test results below the threshold for Type 2. Due to this, it is mandatory to measure BEC and FeNO on at least three occasions, or at least 1–2 weeks after OCS/on the lowest possible OCS dose.7
Mepolizumab Clinical Trial Data
REALITI-A is a global, prospective observational cohort study evaluating the real-world effectiveness and safety of mepolizumab in patients with severe asthma (N=822). A post-hoc analysis evaluated 1-year outcomes stratified by comorbidities at enrolment. It was observed that the reduction in severe exacerbations versus baseline was 75% for patients suffering from nasal polyps and 69% for those with asthma alone.31
The post-hoc analysis also demonstrated the potential for OCS freedom in patients. Among patients with severe asthma, 46% of those with comorbid CRSwNP (n=104) and 40% of those without CRSwNP (n=118) were able to eliminate maintenance OCS from their treatment schedule after 1 year of treatment. 31
Case Study 2
Werminghaus shared a hypothetical patient case from his perspective as an ear, nose, and throat (ENT) specialist, of a 52-year-old engineer, diagnosed with CRSwNP at the age of 48 years. Over the past 4 years, her condition has worsened, marked by frequent, acute exacerbations of CRSwNP. She reports symptoms including nasal congestion, post-nasal drips, facial pain, infections of the airways, frequent shortness of breath, and nighttime symptoms. Two years ago, she underwent sinus surgery. Her current treatment includes low-dose ICS/LABA, a salbutamol inhaler, and mometasone furoate, and she suffers from gastro-oesophageal reflux disease and asthma. Werminghaus commented: “As an ENT surgeon, 10 years ago, I would think about surgery, but it is no longer that time. It’s 2025, and we investigate more… I investigate the lungs, perform a CT scan, assess symptoms, and current medication… and besides surgery, we consider biologics.”
Phase III SYNAPSE Study
In the Phase III SYNAPSE study, a randomised, double-blind 52-week study (N=407), mepolizumab demonstrated significant dual benefit in patients with upper and lower airway disease.32 By Week 52, a greater proportion of patients with CRSwNP had experienced a ≥1-point or ≥2-point improvement from baseline in total endoscopic NP score compared with placebo. This is also supported by real-world evidence.33 A 2022 retrospective, observational, multicentre real-life study evaluated patients with severe allergic asthma and nasal polyps treated by benralizumab, mepolizumab, or omalizumab for 6 months (N=72). After 6 months, all patients experienced benefit in asthma symptoms, FEV1 scores, asthma control test, and reduced NP score and BEC.33
In a hypothetical patient, biologic treatment may alleviate nasal congestion,34 steroid burden,32 sleep disruption and fatigue,34 work impairment,34 and activity impairment.34 Werminghaus emphasised that when treating patients with CRSwNP and severe asthma, we should consider everything as one airway, one disease.
Safety Data
In clinical trials, mepolizumab had a similar incidence of adverse events versus placebo (Table 1), except for injection site reactions (8% versus 3%), occurring mainly within the first three injections.35,36 Mepolizumab has 10 years of long-term extension safety data in severe asthma with Type 2 inflammation.37
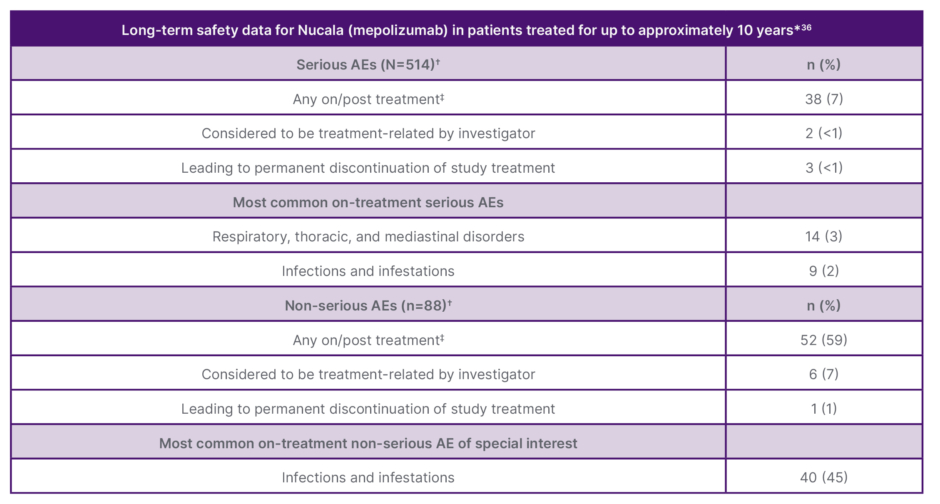
Table 1: Long-term safety data for patients treated with mepolizumab for up to approximately 10 years.36
*10 years based on a multicentre, open-label, LTE safety study of patients treated with Nucala for a maximum of 9.97 years, including 6.44 years in the LTE and the previous years in Nucala clinical and open-label trials, inclusive of interruptions in dosing with a given study.
✝Serious AEs were assessed in all 514 patients in the study. Non-serious AEs were assessed in a smaller sub-group of 88 patients.
‡For serious AEs: any on-treatment, 34 (7%); any post-treatment, 1 (<1%). For non-serious AEs: any on-treatment, 43 (57%); any post-treatment, 2 (3%).
AE: adverse events; LTE: long-term extension.
Adapted from Pavord et al.36
Conclusion
CRSwNP poses a high disease burden to patients, particularly among patients with comorbid severe asthma, affecting multiple dimensions of daily living and quality of life. Recent evidence points towards a multidirectional effect of IL-5 in T2 inflammation, which offers potential for disease modification in patients with asthma and CRSwNP. Data presented on mepolizumab, an anti-IL5 monoclonal antibody, demonstrated durable clinical efficacy with a favourable safety profile over approximately 10 years of treatment, supported by both clinical trial data and real-world evidence.
![]()
UK Prescribing Information can be found here.
| Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow card in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221 441. |
Nucala (mepolizumab) is indicated as an add-on treatment for severe refractory eosinophilic asthma in adults, adolescents and children aged 6 years and older. Chronic rhinosinusitis with nasal polyps (CRSwNP): indicated as an add-on therapy with intranasal corticosteroids for the treatment of adult patients with severe CRSwNP for whom therapy with systemic corticosteroids and/or surgery do not provide adequate disease control. Eosinophilic granulomatosis with polyangiitis (EGPA): indicated as an add-on treatment for patients aged 6 years and older with relapsing-remitting or refractory eosinophilic granulomatosis with polyangiitis. Hypereosinophilic syndrome (HES): indicated as an add-on treatment for adult patients with inadequately controlled hypereosinophilic syndrome without an identifiable non-haematologic secondary cause.
For Healthcare Professionals outside of the UK, please refer to your Local Prescribing Information and Adverse Event Reporting Guidelines.







