Article Summary
Spacers or valved holding chambers added to pressurised metered dose inhalers (pMDI) reduce errors in inhaler technique and improve delivery of fine dose particles to patients with obstructive airway diseases. However, despite the established benefits of spacers, patients frequently do not use them beyond the home setting due to inconvenience regarding their relatively large size and appearance. Notably, not using the spacer (i.e., nonadherence) could have negative consequences for more than just the patients themselves.Alan Kaplan, Physician and Chairperson of the Family Physician Airways Group of Canada, Markham, Ontario, Canada; and Job F.M. Van Boven, Associate Professor of Cost-Effective & Sustainable Respiratory Drug Use, University Medical Center Groningen, the Netherlands, explored the intricate relationships between adherence to prescribed treatments with spacers, symptom control and management, the carbon footprint, and economic burden on the healthcare system. They also introduced a novel, portable spacer that was designed to be used while on the go. Specifically, they discussed the performance of the portable spacer compared to the gold standard traditional spacer, pMDIs alone, and dry powder inhalers (DPI). Finally, summarising patient feedback and adherence data about the new portable spacer.
INTRODUCTION
Valved holding chambers, commonly referred to as spacers, are medical devices used with a pMDI to improve the delivery of medication to the lungs. They are an important aspect of the pMDI delivery system used by a large share of patients with asthma and/or COPD. Spacers have been shown to increase lung deposition, reduce oropharyngeal deposition, and make it easier for people of all ages to breathe in their inhaler medicine by overcoming challenges with poor inhalation technique.1,2
Despite the crucial role that spacers play in optimising the delivery of inhaled medicines via pMDIs, most patients do not use spacers with their inhalers.3 For those who use a spacer, many are prone to leaving them at home when on the go due to their size and appearance.3 In this expert interview, Kaplan and Van Boven describe the clinical, economic, and environmental impact of spacer use,4 and explore innovations in spacer design that aim to optimise the effects of inhaled medicines.
EFFICIENT MEDICATION DELIVERY: GOOD FOR THE PATIENT, THE ECONOMY, AND THE ENVIRONMENT
Where Are Patients Likely to Experience Exacerbations, and What Do Guidelines Recommend for Symptom Control and Management?
One of the main inhaler treatment goals of asthma and COPD is to avoid and cope with the occurrence of exacerbations, i.e., periods with worsening and sometimes life-threatening symptoms, including severe dyspnoea, cough, and sputum production. Exacerbations often occur outside the home when people are exposed to different triggers, such as weather, pollen, pollution, and fragrances. Even people whose symptoms are typically mild and well-controlled can experience an exacerbation with little warning.5 Inhaler medication can be difficult to inhale in an emergency, especially when specific flow rates and coordination are required. During exacerbations, people are often breathless, coughing, and even panicking, which can impede effective use of inhaled therapy at the time when it’s needed most.5 As such, the European Respiratory Society (ERS) recommends that people have a pMDI and spacer emergency treatment pack for self-management of exacerbations, especially if using DPIs for regular treatment.6
How Does Spacer Use Relate to the Economic Burden on the Healthcare System?
Directly, spacers allow for more efficient delivery of pMDI doses, reducing the need for treatment of side effects (e.g., oral thrush) and the likelihood of requiring additional doses, due to symptoms being controlled with less medication.4 Indirectly, if spacers are not used and patients’ symptoms are not controlled at standard doses, clinicians may deem this as intrinsically ineffective medication (while the actual underlying reason is incorrect inhaler usage). For this indirect case, clinicians may unnecessarily step up treatment to more expensive medications (e.g., biologics) that may cost up to 30 times more than inhaler treatment.7
How Does the Use of Spacers Relate to the Carbon Footprint of Treatment with Aerosolised Medication?
Despite their clinical effectiveness, the propellants currently used in pMDIs (hydrofluoroalkane [HFA]-134a and HFA-227) can have a negative impact on the environment due to their global warming potential. The development of greener propellants (HFA-152a and HFO-1234ze) is one of the key strategies to reduce the environmental impact of pMDIs, which will still benefit from use with spacers. Additionally, using current pMDIs with low propellant volume along with a spacer has shown the potential to improve medication delivery and reduce carbon emissions.8
Moreover, the ERS, British Thoracic Society (BTS), and Canadian Thoracic Society (CTS) are all encouraging the use of spacers as an important strategy to reduce greenhouse gas emissions from pMDIs through more efficient medication delivery.6,9,10
Notably, more efficient delivery of pMDI doses with a spacer reduces the total number of reliever doses and may decrease the likelihood of further deterioration and the need for emergency room or hospital visits, which have been associated with high environmental impact.4,11 Indeed, optimal respiratory control will simultaneously minimise environmental burden and improve the quality of patient care, which has previously been described by Usmani and Levy in 2023,12 who said: “The most appropriate and environmentally friendly inhaler is one that a patient will adhere to and use correctly: this minimises wastage and promotes good disease control.”
Additional Context: Overcoming the Portability Challenge of Spacers
To overcome the portability challenges related to spacer usage, the AeroChamber2go* Spacer (Trudell Medical International, London, Ontario, Canada) was recently introduced, specifically designed for use on the go. Of note, throughout its development process, people with asthma and COPD were included to ensure the device met their needs for usage outside the home environment. The patient-centric design process likely contributed to the high patient satisfaction and increased adherence demonstrated with the new AeroChamber2go* Spacer device.11,13,14 The device has a compact, portable, and consumer-friendly form, conceived to encourage patients to carry the device with them outside the home. It dually functions as a protective case for the inhaler, made from durable, shatter-resistant material, with an easy-to-use opening mechanism that allows patients to easily access their inhaler in emergency situations. In addition, the chamber is manufactured sustainably using locally sourced components and over 90% green energy sources for electricity, resulting in a very low global warming potential (Trudell Medical International, data on file).
EVALUATING ON-THE-GO SPACER PERFORMANCE
What Performance Evaluations Have Been Conducted with the AeroChamber2go* Spacer, and with Which pMDIs?
Several laboratory studies have assessed the in vitro performance of the AeroChamber2go* Spacer. These investigations were carried out with commonly used relievers, as well as increasingly prescribed combination inhalers such as maintenance and reliever therapies, and anti-inflammatory reliever therapies. The list of pMDIs evaluated to date includes: AirSupra® (AstraZeneca, Cambridge, UK), Fostair® (Chiesi, Parma, Italy), Symbicort® (AstraZeneca, Cambridge, UK), Ventolin® HFA (GSK, London, UK), Atrovent® (Boehringer Ingelheim, Ingelheim am Rhein, Germany), Salamol® (Norton Healthcare Ltd, Essex, UK), and Teva Salbutamol (Teva Pharmaceutical Industries Ltd, Tel Aviv, Israel).15-21
Improved performance compared to the use of a pMDI alone
Use of the new portable spacer was shown to provide similar or improved delivery compared to a pMDI alone (even when the pMDI was used with perfect technique; Figure 1).15-18,20,21 In addition, the spacer significantly reduced the delivered coarse particle dose compared to the use of pMDI alone.15-17 The reduction of these large aerosol particles is important because they can potentially lead to side-effects such as thrush, hoarseness, sore throat, and potentially even poor oral health and dental effects.1,22-24 This is particularly important with new maintenance and reliever/anti-inflammatory-reliever therapies, where inhaled corticosteroids would be inhaled on the go, and people may not be able to rinse their mouths immediately after use as recommended.
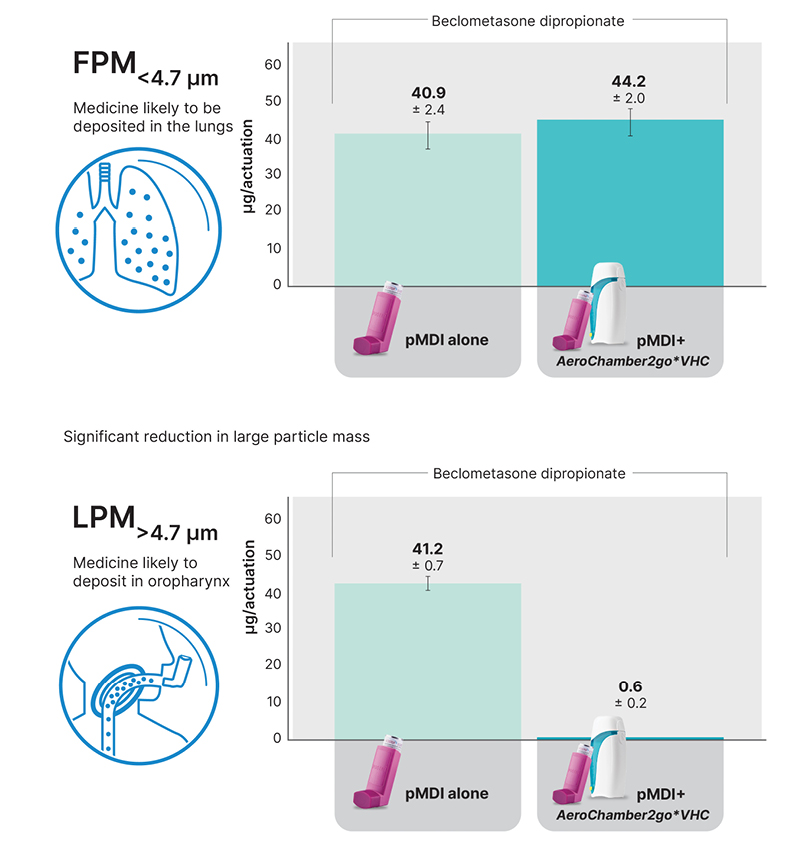
Figure 1: Fine particle mass and large particle mass for inhaled corticosteroid component of Fostair® metered dose inhaler (100/6).16
AeroChamber2go*: Trudell Medical International, London, Ontario, Canada; Fostair®: Chiesi, Parma, Italy; FPM: fine particle mass; LPM: large particle mass; pMDI: pressurised metered dose inhalers; VHC: valved holding chamber.
Similar performance as widely prescribed AeroChamber Plus* Flow-Vu* Spacer
To demonstrate non-inferior performance to the gold standard spacer, studies have compared the AeroChamber2go* Spacer with the widely prescribed AeroChamber Plus* Flow-Vu* Spacer (Trudell Medical International, London, Ontario, Canada). The studies showed similar quantities of fine particle mass (<4.7 µm), indicative of potential lung delivery.15,18
Improved performance compared to dry powder inhaler
A laboratory study compared the performance of the Easyhaler® DPI (Orion Corporation, Espoo, Finland) to the Teva Salamol® pMDI when used in conjunction with the AeroChamber2go* Spacer. The pMDI/Spacer combination was more efficient at delivering medication at all flow rates.19 In addition, the coarse particle dose was reduced by approximately nine times compared to the DPI at optimal flow rates, and even more at suboptimal rates.25
How Has the Portable Device Been Shown to Impact Patient Adherence and Satisfaction?
A survey was completed by 86 users in Canada who were using a traditional spacer and then started using the AeroChamber2go* Spacer to understand the users’ perspective of the portable device. Overall, highly positive impressions were reported: 99% were very or extremely satisfied with ease of use, 97% found the device easy to carry, 95% reported effective delivery, and 92% were favourable to the aesthetics.13 Further, the percentage of patients using a spacer outside the home doubled from 39% to 86% (Figure 2).13
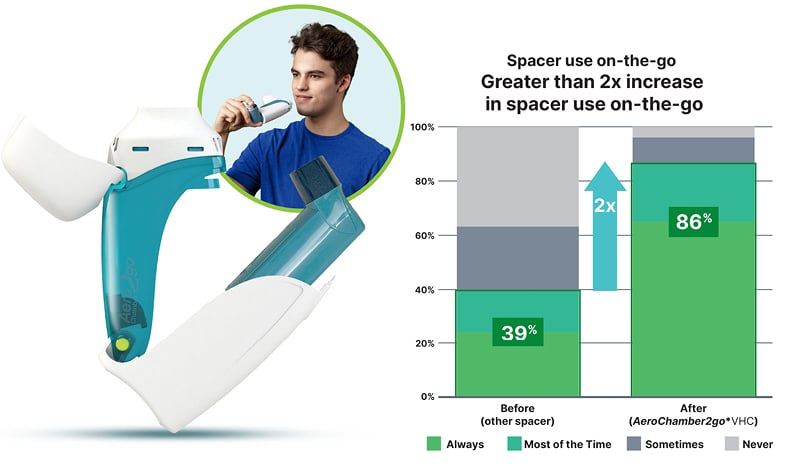
Figure 2: Improved adherence on-the-go compared to traditional spacer device.14
AeroChamber2go*: Trudell Medical International, London, Ontario, Canada; VHC: valved holding chamber.
A similar preference study was completed in the Netherlands. Results showed that patients with asthma/COPD found the novel compact spacer generally acceptable, with 85% of people reporting being highly likely to continue using it.14
What Link Has Been Demonstrated Between Improved Patient Outcomes, the Healthcare System, and the Environment?
In a Canadian survey (N=409), 91% of people reported using the AeroChamber2go* Spacer all or most of the time. Compared to using a pMDI alone, 82% were more confident in medication delivery, 26% noted fewer puffs were required, 11% noticed fewer side effects, and 9% reported fewer emergency visits.11
With many of these outcomes being contributors to carbon emissions, these findings suggest the new on-the-go spacer could be beneficial to the patient, the healthcare system, and the environment (Figure 3).26
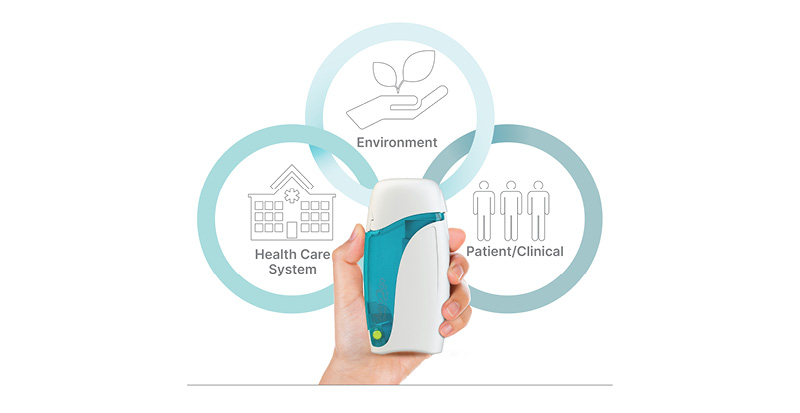
Figure 3: Portable spacer benefits patient, healthcare system, and environment.11
CONCLUSION
In this expert interview, Kaplan and Van Boven described how efficient medication delivery using spacers is recognised as an integral aspect of the pMDI delivery system, especially with increasing recommendations for more expensive combination and biologic therapies and environmental concerns regarding the currently used propellants in inhalers. They highlighted that these issues are intrinsically linked. Optimal respiratory control, including through the use of devices like spacers, is an important strategy to minimise environmental burden and improve the quality of patient care. However, implementation depends on patients being adherent to their respiratory care plans, including using their spacers outside the home.
Kaplan and Van Boven also introduced the novel portable AeroChamber2go* Spacer, which presents a viable device for use when patients are on the go, and demonstrates potential benefits for patients, the healthcare system, and the environment.







