Abstract
Objective: To evaluate the diagnostic value of prostate-specific antigen (PSA) density, digital rectal examination (DRE), and family history in predicting prostate cancer among patients with low-risk Prostate Imaging Reporting and Data System (PI-RADS) 1 and 2 lesions who underwent prostate biopsy.
Methods: The authors retrospectively analysed 153 patients with PI-RADS 1–2 lesions who underwent systematic and/or targeted transrectal ultrasound-guided prostate biopsy at a tertiary urology centre between 2022–2024. Clinical parameters including PSA level, PSA density, prostate volume, DRE findings, and family history were recorded. Cancer detection rates and significant predictors were identified using univariate analysis and logistic regression. Diagnostic performance was assessed with receiver operating characteristic curve analysis.
Results: Prostate cancer was detected in 16/153 patients (10.5%). Patients with cancer had higher PSA density, more frequent abnormal DRE findings, and a significantly higher rate of positive family history. Logistic regression revealed that abnormal DRE (odds ratio: 0.062; p=0.009) and family history (odds ratio: 0.211; p=0.014) were independent predictors of cancer detection. The combined model showed moderate diagnostic performance (area under the curve: 0.711; p=0.006). PSA density showed a trend towards significance (p=0.082), but did not reach statistical significance independently.
Conclusion: Although PI-RADS 1 and 2 lesions are typically considered low-risk, the authors’ findings suggest that clinical factors such as suspicious DRE and family history significantly enhance the detection of prostate cancer in this population. Incorporating these variables into biopsy decision-making may help avoid missed diagnoses and support a more individualised approach in patients with low-risk imaging findings.
Key Points
1. A notable proportion of patients with Prostate Imaging Reporting and Data System (PI-RADS) 1–2 lesions may still harbour prostate cancer despite low-risk imaging.2. Digital rectal examination and a positive family history are significant clinical predictors of cancer detection in this group.
3. Incorporating clinical risk factors into biopsy decisions can improve diagnostic accuracy and prevent missed diagnoses.
INTRODUCTION
Prostate cancer remains one of the most prevalent malignancies among men worldwide, underscoring the need for effective diagnostic strategies to facilitate early detection and inform appropriate clinical management.1 Multiparametric MRI (mpMRI) has become an essential component in the diagnostic pathway, enhancing the detection of clinically significant prostate cancer (csPCa) while reducing the number of unnecessary biopsies.2 To standardise mpMRI interpretation, the Prostate Imaging Reporting and Data System (PI-RADS) was developed, enabling the categorisation of prostate lesions based on their likelihood of harbouring csPCa.3
While PI-RADS 3–5 lesions are frequently biopsied due to their stronger association with malignancy, the management of PI-RADS 1 and 2 lesions remains a clinical challenge.4 These low-score lesions are generally considered to have minimal malignant potential, often resulting in conservative management. However, biopsies are still performed in selected patients with PI-RADS 1–2 findings, particularly when additional risk factors, such as elevated prostate-specific antigen (PSA) levels, abnormal digital rectal examination (DRE) findings, or a positive family history, are present. This highlights the need for more precise and evidence-based criteria to guide biopsy decisions in this subgroup.5
Previous studies have primarily focused on the predictive value of PI-RADS 3–5 lesions, with limited data available regarding the clinical significance of PI-RADS 1 and 2 findings.5 The absence of clear guidelines for biopsy in this lower-risk population may result in the underdiagnosis of clinically relevant prostate cancer or the performance of unnecessary invasive procedures. Thus, refining the selection criteria for biopsy in patients with PI-RADS 1–2 lesions is essential for optimising diagnostic accuracy and reducing overtreatment.
In this study, the authors aimed to identify variables associated with prostate cancer detection in patients with PI-RADS 1 and 2 lesions who underwent biopsy despite their low-risk imaging classification. By evaluating factors such as PSA density, DRE findings, prostate volume, and family history, the authors seek to contribute to a more individualised and evidence-based approach to biopsy decision-making in this patient population.
MATERIALS AND METHODS
The study included male patients who underwent mpMRI for suspected prostate cancer, were assigned a PI-RADS 1 or 2 score, and subsequently underwent prostate biopsy due to persistent clinical suspicion. Data were retrospectively collected from medical records between 2022–2024.
Inclusion criteria comprised patients with mpMRI-detected PI-RADS 1 or 2 lesions who underwent systematic and/or targeted prostate biopsy and had complete clinical and pathological data available. Patients were excluded if they had a prior history of prostate cancer, previous prostate interventions or treatments, active urinary tract infection or prostatitis at the time of biopsy, or insufficient imaging or biopsy data. Information on family history of prostate cancer was obtained through patient interviews. A positive family history was defined as having at least one first-degree relative (father or brother) diagnosed with prostate cancer.
The following clinical parameters were recorded: patient age, PSA level, PSA density, prostate volume (measured via transrectal ultrasound), DRE findings, and family history of prostate cancer. Imaging findings were evaluated according to the most recent PI-RADS classification guidelines.
All patients underwent transrectal ultrasound-guided prostate biopsy, with systematic sampling of at least 12 cores. Additional targeted biopsies were obtained when a focal lesion was suspected. Histopathological evaluation categorised biopsy specimens as benign, atypical small acinar proliferation, chronic prostatitis, or prostate cancer. csPCa was defined as a Gleason score ≥7.
Statistical Analysis
Descriptive statistics were calculated for both continuous and categorical variables. Comparisons between cancer-positive and cancer-negative groups were conducted using the independent t-test or Mann–Whitney U test for continuous variables, and the χ2 or Fisher’s exact test for categorical variables. Logistic regression analysis was performed to identify predictors of prostate cancer detection. Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the diagnostic performance of significant variables, and the area under the curve (AUC) was calculated to assess predictive accuracy. A p value of <0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 22.0 (IBM, Armonk, New York, USA).
RESULTS
A total of 153 patients with PI-RADS 1 and 2 findings were included in the analysis. Of the 153 patients who underwent biopsy, 16 (10.5%) were diagnosed with prostate cancer. Of these, seven patients had Gleason 3+3 disease, six had Gleason 3+4, and three had Gleason 4+3. Accordingly, nine out of 16 cancers (56.3%) were clinically significant (Gleason ≥7). The remaining cases were categorised as benign prostatic hyperplasia (n=115; 75.2%), atypical small acinar proliferation (n=11; 7.2%), and chronic prostatitis (n=11; 7.2%).
The mean prostate volume was 70.88±29.98 mL in the cancer-positive group and 87.85±50.03 mL in the cancer-negative group (p=0.493). The mean age was similar between the groups (65.50±7.32 years versus 64.42±6.77 years; p=0.258). PSA levels were also comparable, with 10.86±20.66 ng/mL in patients who were cancer-positive and 7.91±8.28 ng/mL in patients who were cancer-negative (p=0.756). PSA density was higher in the cancer-positive group (0.19560±0.44109) than in the cancer-negative group (0.09450±0.07307), approaching statistical significance (p=0.093). The presence of firm nodules on DRE was significantly more frequent in the cancer-positive group (12.5%) compared to the cancer-negative group (1.5%; p=0.006). All patients who were cancer-positive (100%) had PI-RADS 2 findings. A positive family history of prostate cancer was present in 31% of the cancer-positive group (5/16) and 10.9% of the cancer-negative group (15/137), which was statistically significant (p=0.023). These characteristics are summarised in Table 1.
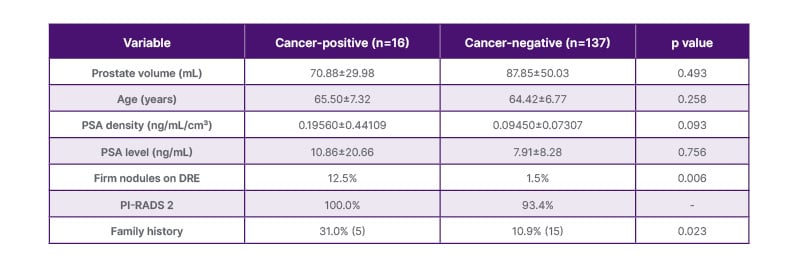
Table 1: Baseline characteristics of patients with and without prostate cancer.
Continuous variables were compared using the independent t-test or Mann–Whitney U test, while categorical variables were analysed using the χ2 test or Fisher’s exact test, as appropriate.
DRE: digital rectal examination; PI-RADS: Prostate Imaging Reporting and Data System; PSA: prostate-specific antigen.
In the logistic regression analysis, the presence of a family history (p=0.014), DRE findings (p=0.009), and PSA density (p=0.082) were included in the model. The presence of a suspicious DRE and positive family history were significant predictors of prostate cancer. Specifically, a suspicious rectal examination reduced the odds of not having cancer (odds ratio: 0.062), and having a family history also decreased the odds (odds ratio: 0.211). The model was statistically significant (Omnibus Test of Model Coefficients: χ²=13.289; p=0.004) and explained approximately 17% of the variance in cancer status (Nagelkerke R²=0.170). The predictive accuracy of the model was 89.5%, correctly identifying 135/137 patients without cancer (specificity: 98.5%), but only 2/16 patients with cancer (sensitivity: 12.5%). These characteristics are summarised in Table 2.
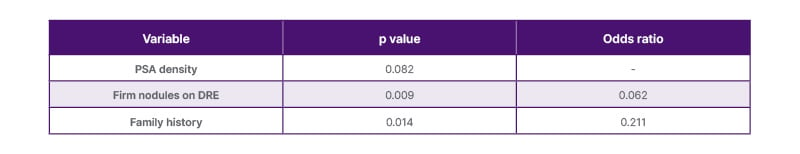
Table 2: Logistic regression analysis of prostate-specific antigen density, digital rectal examination, and family history.
DRE: digital rectal examination; PSA: prostate-specific antigen.
The AUC-ROC for the predicted probability was 0.711 (95% CI: 0.556–0.867; p=0.006), indicating moderate diagnostic performance. The combined predictive probability of PSA density, family history, and DRE findings demonstrated strong diagnostic utility in identifying patients at risk of prostate cancer despite low PI-RADS scores. The ROC curve analysis is shown in Figure 1.
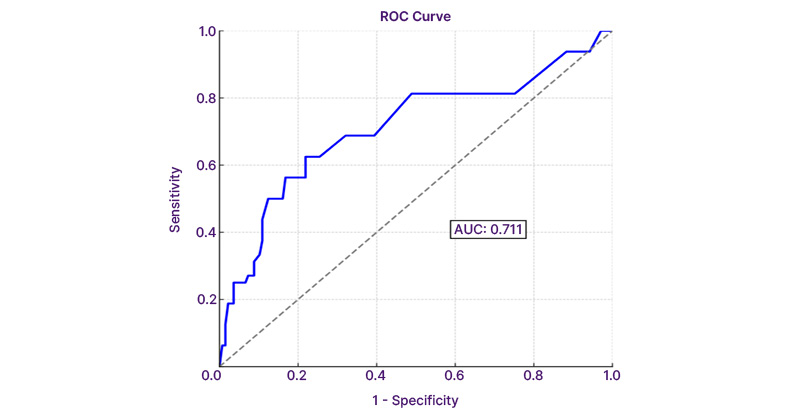
Figure 1: Receiver operating characteristic analysis of combined predictors: prostate-specific antigen density, family history, and digital rectal examination.
Diagonal segments are produces by ties.
Sensitivity: 12.5%; specificity: 98.5%; 95% CI: 0.556–0.867; p=0.006.
AUC: area under the curve; ROC: receiver operating characteristic.
DISCUSSION
The findings of this study provide valuable insights into the factors influencing prostate cancer detection in patients with PI-RADS 1 and 2 lesions who underwent biopsy despite their low-risk imaging classification. The authors’ results highlight the significance of DRE findings and family history in identifying patients at higher risk of prostate cancer, while PSA density showed a trend towards significance. Conversely, prostate volume, age, and PSA levels did not emerge as significant predictors in this cohort.
The role of mpMRI in prostate cancer diagnosis has been well established, with PI-RADS serving as a critical tool for risk stratification.3 While PI-RADS 3–5 lesions are commonly subjected to biopsy due to their higher malignancy probability, there remains considerable uncertainty regarding biopsy indications in patients with PI-RADS 1 and 2 lesions.6 Previous studies have largely overlooked the clinical relevance of this subgroup, often assuming a negligible cancer risk.2 However, the authors’ findings underscore that a subset of these patients still harbour csPCa, necessitating a more nuanced approach to biopsy decision-making.
The authors’ study observed a cancer detection rate of 10.5% in patients with PI-RADS 1 and 2 lesions. This aligns with previous research indicating that, although the likelihood of csPCa is lower in PI-RADS 1 and 2 categories, it is not negligible. For instance, a study reported that deferring biopsy in patients with PI-RADS 1 or 2 lesions could miss up to 16% of Gleason score ≥7 cancers.7 These findings suggest that relying solely on mpMRI findings may lead to the underdiagnosis of significant cancers, highlighting the need for additional clinical parameters to be integrated in the decision-making process.8 This distribution indicates that, while over half of detected cancers were clinically significant, a proportion of low-grade (Gleason 6) cancers was also identified. This suggests that biopsy decisions in patients with PI-RADS 1–2 lesions can uncover both indolent and aggressive disease.
Current guidelines recommend considering biopsy deferral in patients with PI-RADS 1 or 2 lesions, especially when other risk factors are absent. The authors’ study reinforces the importance of a comprehensive clinical assessment, including DRE findings and family history, to identify patients who may benefit from a biopsy despite low-risk imaging results. This approach aligns with the guidelines, which emphasise individualised risk assessment in prostate cancer diagnosis.9
DRE findings were significantly associated with prostate cancer detection, reinforcing the importance of incorporating clinical examination into biopsy decision algorithms.10 This aligns with prior research emphasising the predictive value of abnormal DRE findings, even in the presence of low-risk mpMRI findings.11 Additionally, the presence of a positive family history markedly increased the likelihood of cancer detection, suggesting that genetic predisposition remains a crucial factor independent of imaging findings.12 Several genome-wide association studies have identified multiple susceptibility loci associated with an increased risk of prostate cancer, including genes such as BRCA1, BRCA2, HOXB13, and variants on chromosome 8q24.13 These findings emphasise the importance of genetic screening in high-risk individuals, particularly those with a strong family history. Given these results, clinicians should maintain a high index of suspicion in patients with suspicious DRE findings or a strong family history, even when PI-RADS classification suggests a low probability of malignancy.
PSA density has been proposed as a useful adjunct in biopsy decision-making, with prior studies indicating its potential role in refining risk assessment.14,15 In the authors’ study, PSA density demonstrated a trend towards significance, suggesting that it may contribute to risk stratification in patients with PI-RADS 1–2 lesions, but may not be a standalone predictor. Further research with larger cohorts may better delineate its utility in this setting.
The absence of a significant association between prostate volume, age, and PSA level with cancer detection in the authors’ study is noteworthy. While larger prostate volumes have been linked to lower PSA density and reduced cancer risk, the authors’ findings suggest that prostate size alone may not be a decisive factor in patients with PI-RADS 1–2 lesions.16 Similarly, PSA level, although widely used as a screening tool, did not provide additional discriminatory value in this subgroup, reinforcing the need for a multifactorial approach incorporating clinical and genetic risk factors.
The authors’ study has several strengths, including a well-defined patient cohort and a focus on an often-overlooked subset of patients undergoing prostate biopsy. Additionally, the ROC curve analysis demonstrated an AUC of 0.711, indicating good discriminatory power in differentiating between cancer-positive and cancer-negative cases. This suggests that, while PI-RADS 1 and 2 lesions are generally considered low risk, integrating additional clinical parameters such as PSA density, DRE findings, and family history enhances diagnostic accuracy. The strong performance of the authors’ model highlights the potential utility of combining imaging and clinical data in biopsy decision-making. Future studies should validate these findings in larger, multicentre cohorts to establish their broader applicability. However, the study also has limitations. The retrospective design introduces inherent selection biases and the sample size, particularly in the cancer-positive group, remains relatively small. Additionally, while family history was a significant predictor, its precise impact could be influenced by recall bias or incomplete medical records. Future prospective studies with larger sample sizes and genetic profiling may offer more definitive conclusions. The lack of genetic analysis in the authors’ study represents a key limitation, as they were unable to assess the potential contribution of inherited genetic mutations to prostate cancer risk in this cohort. Genetic testing in this context would typically involve germline mutation analysis for genes such as BRCA1, BRCA2, and HOXB13, as well as risk-associated loci like 8q24. Incorporating such testing would allow for a more accurate evaluation of hereditary predisposition and clarify whether the observed effect of family history reflects true genetic susceptibility or reporting bias. Incorporating genetic testing into future studies could provide a more comprehensive understanding of the hereditary components of prostate cancer and refine risk stratification models.
CONCLUSION
In conclusion, the authors’ study suggests that, while PI-RADS 1 and 2 lesions are generally associated with a low probability of malignancy, certain clinical factors warrant consideration when deciding on biopsy. DRE findings and family history emerged as significant predictors of prostate cancer detection, underscoring their role in clinical decision-making. PSA density may offer additional value, but requires further validation. These findings highlight the importance of an individualised, risk-adapted approach to biopsy in patients with low-risk PI-RADS classifications.







