Interview Summary
Hypertrophic cardiomyopathy (HCM) is an inherited disease characterised by thickening of the left ventricular wall, which can lead to symptoms such as fatigue, exertional dyspnoea, and an increased risk of sudden cardiac death. The approval of the first targeted therapy (mavacamten, a cardiac myosin inhibitor [CMI]) to treat obstructive HCM (oHCM) has been a significant advance. However, there are still many therapeutic unmet needs, crucially the lack of an approved treatment for non-obstructive HCM (nHCM). Additionally, CMIs can cause decreases in LVEF that necessitates a resource-intensive dose-titration process. EDG-7500, a novel, selective cardiac sarcomere modulator, is currently in development for the treatment of HCM, and data from the 4-week portion of the Phase II CIRRUS-HCM study of EDG-7500 were presented at the Annual Meeting of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) in May 2025. During the interviews conducted for the European Medical Journal (EMJ) in June 2025, the significance of these new data was discussed by Michelle Michels, Director of the Erasmus MC Center of Expertise for Inherited Cardiovascular Diseases, Rotterdam, the Netherlands; and Perry Elliott, Director of the Institute of Cardiovascular Science, University College London (UCL), and consultant cardiologist at St Bartholomew’s Hospital, London, UK. The experts reviewed the clinical findings of CIRRUS-HCM, highlighting key elements including diastolic benefits and preservation of systolic function, as well as mechanistic features and practicalities, which have the potential to differentiate EDG-7500 from current targeted therapies.THE DEVELOPING TREATMENT LANDSCAPE
HCM is an inherited condition in which hypercontractility of the heart muscle leads to thickening of the left ventricular wall, diastolic and systolic dysfunction, reduced cardiac output, and myocardial ischaemia. Better understanding of HCM at the molecular level has allowed the development of therapies that target the actin–myosin interaction in the cardiac sarcomere to address the underlying hypercontractility. CMIs were the first class of targeted therapies for the treatment of HCM, followed by the first-in-class cardiac sarcomere modulator, EDG-7500, now in development, with the most recent clinical data released from the Phase II CIRRUS-HCM study. In this interview article, cardiology specialists Michelle Michels and Perry Elliott review these latest clinical findings for EDG-7500 and consider the potential role for this novel targeted agent within the field of HCM management.
Michels began by outlining some of the difficulties inherent in treating HCM: “It is a complex condition, with a highly variable presentation, from both a clinical and a genetic standpoint. The phenotypes range from [asymptomatic] carriers of a pathogenic, or likely pathogenic, DNA variant without any hypertrophy, to extreme left ventricular hypertrophy, and patients presenting primarily with heart failure. So that’s the big challenge: it’s one disease with one name, but it comes with many different faces.”
HCM is classified as being either obstructive or non-obstructive, depending on the presence of left ventricular outflow tract (LVOT) obstruction at rest or with provocation. Elliott clarified, “Many people with HCM have limiting symptoms of chest pain, breathlessness, and fatigue. In the majority, symptoms are caused by LVOT obstruction.” As noted above, this results from the thickened left ventricular wall and the phenomenon of systolic anterior motion of the mitral valve, in which the valve is driven forward in ventricular systole such that it touches the septum and blocks the outflow of the heart. Elliot explained that patients with nHCM do not have LVOT obstruction, but these patients may still experience symptoms caused by a stiffened heart muscle and progressive heart failure. He added, that some complications are common to both forms of HCM, including a raised risk of atrial and ventricular arrhythmias. “The major preoccupation of doctors and patients alike over the past 50 years has been that the condition is associated with sudden cardiac death. However, with better risk stratification and protective technology (implantable cardiovascular defibrillators), sudden cardiac death is now quite a rare event,” he said. Consequently, the focus has turned toward diastolic dysfunction, an areas where other therapies have not been as successful.
Summarising the traditional symptomatic treatment approach to HCM, Elliott remarked, “Historically, management for HCM started with drugs that target LVOT obstruction by reducing the contractility of the heart: beta blockers, calcium antagonists, and the anti-arrhythmic drug disopyramide. These drugs can be effective, but we now recognise that many patients don’t respond, or the side effects are very unpleasant and not much has changed until very recently.” However, even with the advent of targeted therapy in the form of CMIs, the experts agreed that the treatment options for non-obstructive disease remain limited. Michels clarified, “We currently lack sufficient medical options to treat patients with nHCM and to relieve them from their symptoms, compared with oHCM, where we not only have medications but also invasive treatment options [to reduce the thickening/obstruction in the heart wall].”1,2
The Targeted Treatment Approach
The arrival of CMIs has been an important advance in the treatment of HCM, with mavacamten currently approved to treat oHCM,3,4 and aficamten awaiting approval.5 Considering the pros and cons of CMIs, Michels commented, “I think the big advantage is that [mavacamten] is the first targeted HCM treatment, and there are robust, placebo-controlled trials. In experienced hands, it’s safe, and [its] efficacy is good. However, a drawback is the extensive patient follow-up, including echocardiography to monitor the [potential drop in] left ventricular ejection fraction (LVEF),3,4 which is a challenge with the current pressure on healthcare systems and resources. It also means we can’t use CMIs in patients who already have a reduced LVEF.” The underlying cause of this effect on LVEF is the potent inhibitory action of the CMIs on the cardiac sarcomere. As Elliott explained, “The CMIs are targeting one of the fundamental molecular mechanisms of the disease. If you look at what’s happening at a cellular level in HCM, the cells are contracting more forcefully than is normal, and the consequence is that it increases the energy requirements of the heart, leading to the secondary phenomenon of hypertrophy. So, it was reasoned that if the hypercontractile phenotype could be reduced at a molecular level, it might reduce the drive to hypertrophy. However, the major Achilles heel of the CMIs is that their effect on contractility is very potent. What you don’t want to do is overshoot, reduce the contraction, and risk precipitating heart failure. This is why we have to monitor the contractile function of the heart, the LVEF, with each dose increment. It means that the intensity and frequency of monitoring when you’re starting CMIs is much greater than for any other existing drugs [for the treatment of HCM].” Michels added, “Another drawback is that [mavacamten] is only proven effective in patients with oHCM. We’ve been disappointed and, to a certain level, also surprised that the topline results from the Phase III ODYSSEY-HCM trial [mavacamten in nHCM; NCT05582395] turned out negative recently.”6
EDG-7500
Against the background of this first wave of targeted therapies, EDG-7500 is a novel, oral, selective cardiac sarcomere modulator, which has been specifically designed to reduce the speed and force of early systole, and improve myocardial relaxation in early diastole, without impacting systolic function.7 It is being developed for the treatment of both oHCM and nHCM. Elliott explained how the modulatory effect of EDG-7500 is potentially different from the inhibitory action of the CMIs, and that this could translate into clinical distinctions: “Like the CMIs, EDG-7500 is working on the contractile function of the heart at the sarcomere level, but what it seems to do is […] reduce the velocity at the beginning of contraction, which is when the mitral valve starts to push forwards [leading to systolic anterior motion and obstruction in the LVOT]. So, it affects contractility, but only in that early part of contraction. The other thing it seems to do is improve diastolic function, which is an important target in HCM, and also has the potential to reduce LVOT obstruction without compromising LVEF to the same degree as CMIs.” Michels shared her thoughts on this novel drug: “While the CMIs inhibit the myosin head, EDG-7500 slows the rate of actin–myosin engagement and also speeds up the rate of disengagement [during diastole], so diastolic parameters may also be improved, and hopefully we won’t see the drop in LVEF.”
CIRRUS-HCM
CIRRUS-HCM (NCT06347159) is a Phase II, multi-part, open-label cohort trial8,9 that has provided the first inpatient data for EDG-7500 in HCM. Consistent with preclinical and Phase I study findings in healthy volunteers,7,10,11 CIRRUS-HCM (Part A) showed that a single oral dose of EDG-7500 in patients with oHCM was associated with robust reductions in LVOT gradient and N-terminal pro-B-type natriuretic peptide (NT-proBNP), without meaningful changes in LVEF.7 Newly released data from CIRRUS-HCM (Parts B and C) on the safety and efficacy of multiple (once-daily) oral doses of 50 or 100 mg of EDG-7500 over 4 weeks in patient cohorts with oHCM and nHCM, were presented at the Annual Meeting of the HFA of the ESC in May 2025. Michels and Elliott gave their considered reactions to these findings.
Looking at the study design and patient population of CIRRUS-HCM, the experts favoured the inclusion of oHCM and nHCM cohorts within the same study. They also remarked on the unusual (for HCM) predominance of female patients in both cohorts (oHCM: 71%; nHCM: 58%), and the high rate of hypertension in the oHCM cohort (65%) at baseline.5,12,13
Efficacy Data
In CIRRUS-HCM, early effects on diastolic function with EDG-7500, as well as improvements in quality-of-life measures (Kansas City Cardiomyopathy Questionnaire-Overall/Clinical Summary Scores [KCCQ-OSS/CSS]), were key findings in the oHCM cohort (n=17). Michels remarked, “There is a really quick response. Already at Week 1, you see improvement [from baseline] in LVOT gradient, a drop in NT-proBNP (Figure 1), and also an increase in e’ velocity, which is a diastolic parameter. I think that is impressive. Then at 4 weeks, 89% of the patients [in the 100 mg group] had large/very large improvements in KCCQ-CSS (≥10 points; Figure 2) and 78% had improved their New York Heart Association (NYHA) functional class, which is also a quick response. We know from mavacamten, which has a longer half-life,3,4 that it takes a little bit longer to see these results.”5,12 Elliott’s impressions were also cautiously positive: “It’s a small number of patients, but it’s shown that the drug can reduce LVOT obstruction, both at rest and with Valsalva provocation, and there were reductions in NT-proBNP, which is a very sensitive marker for myocardial strain. So, in that respect, these effects are all very similar to the effects of CMIs.” Echoing Michels’ observations, Elliott also highlighted the magnitude of improvement in quality-of-life score with EDG-7500 in the oHCM cohort.
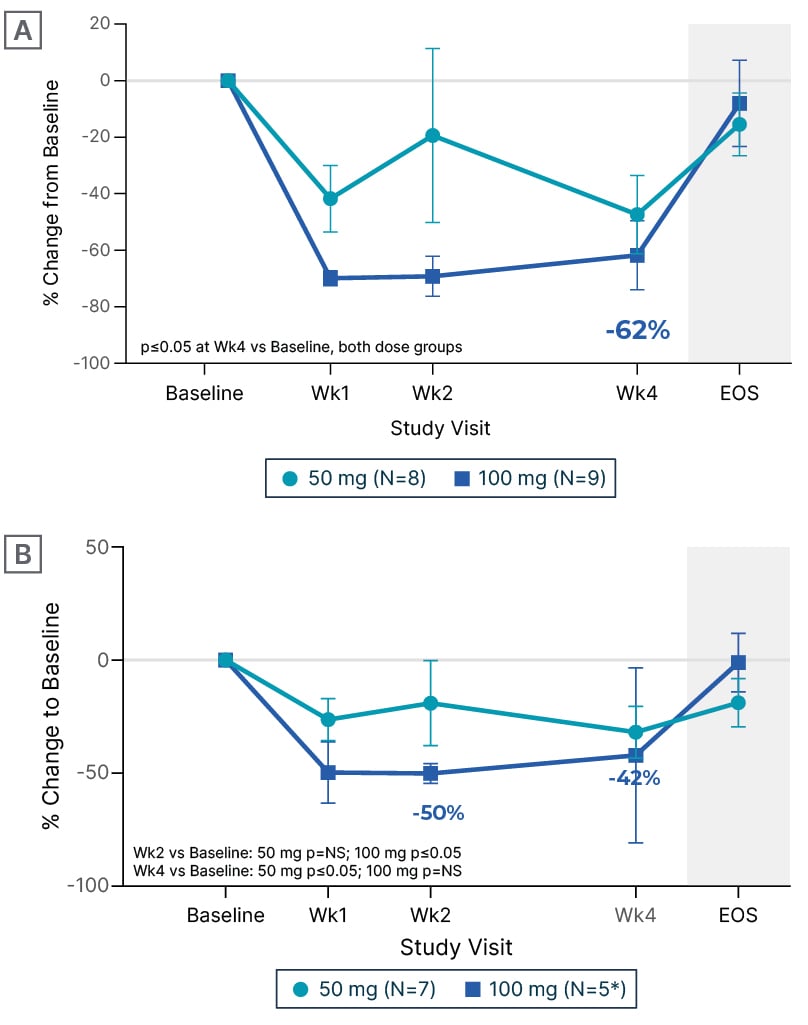
Figure 1: Change from baseline in N-terminal pro-B-type natriuretic peptide with EDG-7500 treatment (CIRRUS-HCM).
A) Obstructive hypertrophic cardiomyopathy. B) Non-obstructive hypertrophic cardiomyopathy.
*Two participants at Week 4.
Data are mean±SEM.
EOS: end of study; NS: non-significant; SEM: standard error of mean; vs: versus; Wk: week.
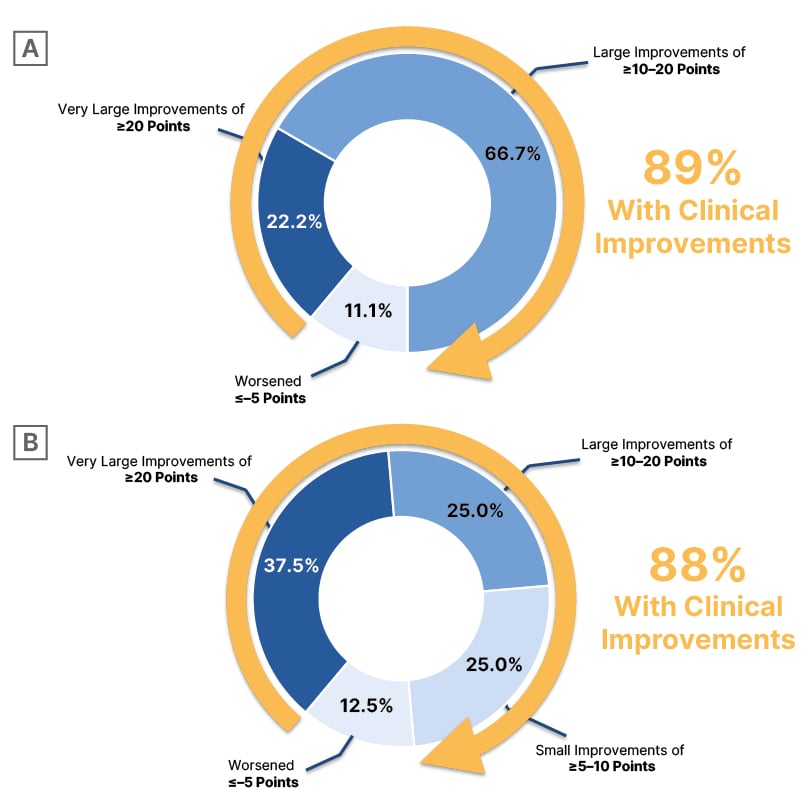
Figure 2: Change from baseline in patient-reported health status (Kansas City Cardiomyopathy Questionnaire-Clinical Summary Score) with EDG-7500 treatment (CIRRUS-HCM).
A) Obstructive hypertrophic cardiomyopathy* B) Non-obstructive hypertrophic cardiomyopathy✝
*100 mg treatment group.
✝Combined 50 mg and 100 mg treatment groups.
In the nHCM cohort (n=12), Michels again noted the rapid and robust effects on NT-proBNP (Figure 1) and e’ velocity, already apparent at Week 1. Elliott described the reduction in NT-proBNP an improvement in diastolic parameters as “reducing one of the fundamental problems in non-obstructive disease,” and explained the significance of the improvements in quality of life seen in both cohorts: “On the KCCQ, a 5-point change is generally regarded as being clinically meaningful. With the CMIs, we see 10–15-point changes [in oHCM], which is pretty dramatic, but in the EDG-7500 data, we see even more change, around 20 points [in KCCQ-CSS for oHCM; 22 points for nHCM; 100 mg groups], which is huge. You certainly wouldn’t see that magnitude of change in heart failure trials with conventional drugs.” Michels was in agreement: “If you look at the obstructive cohort, you see a really impressive improvement in KCCQ-CSS (Figure 2), with 22% of patients [in the 100 mg group] even showing an improvement of ≥20 points. For the nHCM cohort, it’s a little bit more of a mixed bag: some patients respond extremely well, almost 38% [in the combined 50/100 mg groups have improvements of ≥20 points], but then 25% have just small improvements [of ≥5–10 points], but these are still clinically meaningful improvements. We physicians worry about prognosis and the long term, while patients just want to feel better, at least at the start. So, I was impressed by the KCCQ data.”
Summarising their thoughts on the CIRRUS-HCM efficacy data, the experts said that EDG-7500 appeared at least as effective as the CMIs in oHCM, producing similar improvements but with a potentially sooner response. As for nHCM, Michels remarked, “We have just learned that the Phase III ODYSSEY-HCM trial of mavacamten is negative in nHCM6 and we are awaiting the results of the Phase III ACACIA-HCM study of aficamten.14 The aficamten Phase II data look promising (REDWOOD-HCM),15 but I think we have to be careful, because we also thought that the Phase II data for mavacamten looked promising.”16 Consequently, Michels emphasised the importance of the study findings for EDG-7500 in the non-obstructive cohort of CIRRUS-HCM.
Left Ventricular Ejection Fraction Preservation
As noted above, systolic dysfunction leading to reductions in LVEF has been described with the CMIs, making LVEF a key outcome of interest for EDG-7500. “In all drugs that modulate the actin–myosin interaction, we’re afraid of drops in LVEF,” said Michels, “but no meaningful reductions in LVEF were reported in CIRRUS-HCM (Figure 3), and also no LVEF below 50%. That’s very reassuring, although we need to take into account that we are looking at small patient numbers, so it needs to be proven in a larger cohort.” Elliott added that there was no evidence of an LVEF dose-exposure relationship, and suggested that the preservation of LVEF “may be an even greater discriminant from the CMIs in non-obstructive disease.”
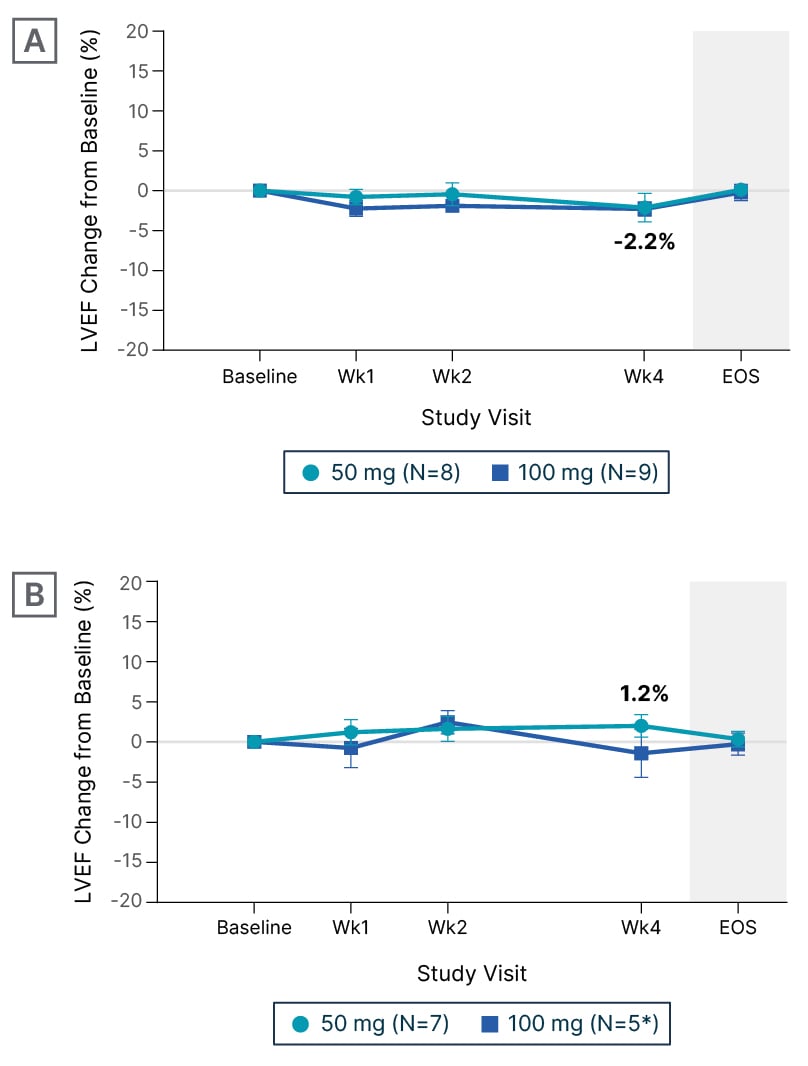
Figure 3: Change from baseline in left ventricular ejection fraction during treatment with EDG-7500 (CIRRUS-HCM).
A) Obstructive hypertrophic cardiomyopathy. B) Non-obstructive hypertrophic cardiomyopathy.
*Two participants at Week 4.
Data are mean±SEM.
EOS: end of study; LVEF: left ventricular ejection fraction; SEM: standard error of mean; Wk: week.
Alongside the important safety implications of LVEF preservation observed with EDG-7500, the experts were keen to emphasise the potential practical benefits of a treatment that requires no LVEF monitoring. “It would be a major advantage for patients first, but also for the healthcare system,” said Michels, with Elliott adding, “At the moment, there are significant restrictions on centres that are allowed to prescribe CMIs. We also have to follow tight protocols for regular monitoring, and there’s no doubt in my mind that this is limiting access to the CMIs, certainly within the UK, but I think in many other European systems as well. We’re having to set up services specifically for the tight up-titration and monitoring of people treated with CMIs, which is a challenge. If we had a drug which we could start and then see the patient at realistic intervals without having to worry about safety echocardiograms, I think that would be a huge step forward.”
Adverse Events
Overall, the experts found EDG-7500 to be generally well tolerated in CIRRUS-HCM in the combined oHCM and nHCM cohorts. The lack of any meaningful reduction in LVEF was viewed as a key differentiating safety outcome (see above), the adverse event profile was largely as expected. Michels noted that, similar to the CMIs, the most common treatment-emergent event was dizziness [27.6%; 8/29 patients], which was mainly mild and transient, and that atrial fibrillation (AF) was reported by 13.8% [4/29 patients]. Elliott commented, “The rate of AF was much higher than seen in the pivotal trials with CMIs,5,12,13,17,18 but this is where small patient numbers are really important. Unfortunately, there were a couple of patients who probably shouldn’t have been included in CIRRUS-HCM. For example, one had mitral valve stenosis, which is a disease where the incidence of AF is very high. I think a fairer comparison would be with the Phase II trials of CMIs; for example, PIONEER-HCM with mavacamten, where the rate of AF was actually very similar to this (27%).19 Obviously, the only way of answering this is through more data, but I’m not particularly concerned by the AF rate at this stage.” Michels added, “There’s been a lot of debate about AF in HCM patients treated with CMIs,20,21 and whether these drugs are increasing the risk of AF, or if we’re just looking at the high prevalence of AF in HCM patients. I don’t think the case is closed on that.”
LOOKING AHEAD
Although cautious about drawing conclusions from Phase II data with small patient numbers (and noting that Part D of CIRRUS-HCM is ongoing), Michels and Elliott were optimistic about the prospects for EDG-7500 and reflected on its future development in HCM. “The next step is obviously a Phase III trial,” said Elliott, “and the best outcome would be that it replicates the results of the Phase II study, so that we’ll have a drug that is safe and capable of reducing obstruction and improving symptoms in obstructive and non-obstructive disease. Then the fundamental question will be, is this better than the CMIs? In an ideal world, we’d like to compare them head-to-head, but I don’t think that’s going to happen. So, the debate will then be around tolerability. Another difference is that the only licensed CMI (mavacamten) has a lot of drug interactions,3,4 and is metabolised in the liver, so if you carry a particular genetic variant in one of your liver enzyme systems, then you have to limit the dose. You don’t have to do that for aficamten18 and, as I understand it, it wouldn’t be an issue with EDG-7500 either. In essence, I think it’s going to be efficacy, safety, [and] impact on quality of life, and if these seem to be better than the CMIs, then EDG-7500 will have the edge. If I were being highly optimistic, I would say that the promise of EDG-7500 is that we could treat obstruction without the worrying side effect of low ejection fraction, and we may have something which is more effective in non-obstructive disease.” Michels concurred, expressing that the big opportunity for EDG-7500 lies in nHCM, where the “playing field is completely open”.
A profile of favourable efficacy with no meaningful impact on LVEF could broaden the population for targeted treatments in HCM without the practical drawback of frequent monitoring visits. As the experts discussed earlier, the need for frequent monitoring and tightly regulated up-titration at an expert medical centre currently limits patient access to mavacamten, so a treatment that could be safely administered beyond Centres of Excellence would favour patient accessibility. Drug–drug interactions and dosing frequency were cited as further important considerations for any emerging treatment, although Elliott observed that there may be a ‘trade-off’ between dosing frequency and feeling better, with the difference between once- or twice-daily dosing becoming less significant to patients if the treatment ‘transforms their lives’. In addition, the experts advocated the benefits of a simple dose titration scheme and noted that intra-patient dose titration for EDG-7500 would be explored in the ongoing CIRRUS-HCM study, with longer-term data expected. Elliott commented, “A more granular dosing regime, adding a lower and a higher dose, would allow us to find the ‘sweet spot’ in efficacy for EDG-7500. This would, ultimately, give us more flexibility in the use of the drug, should clinical findings be positive.”
CONCLUSION
In conclusion, Michels commented, “I think mavacamten, the first of the CMIs, was already a really sophisticated drug, and it’s very promising that there are new drugs coming onto the market that could be even smarter.” Elliott shared this sense of optimism: “The past 5 years have seen such a rapid pace of development in new therapies for people with HCM. We have one new class of drugs (CMIs), and here we are exploring another class. Already, the companies are developing a second wave of drugs, which may tidy up some of the side-effect profiles. This is also occurring in the context of exciting approaches around gene therapy; for example, trying to treat the underlying cause of the disease. So, I think there are a lot of reasons to be optimistic about the future for people with HCM.”







