BACKGROUND AND AIMS
The Norwood procedure for hypoplastic left heart syndrome is one of the most complex neonatal surgeries. Even after technically successful repair, patients remain vulnerable to haemodynamic instability, which can compromise survival and long-term outcomes. Traditionally, clinicians empirically target a pulmonary-to-systemic flow ratio (Qp/Qs) of 1.0 to achieve balance between systemic and pulmonary circulations. However, the true optimal Qp/Qs in the early post-operative period has not been determined.1
This study developed patient-specific computational fluid dynamics (CFD) models of the Norwood circulation using perioperative clinical data. By integrating lactate levels as a marker of haemodynamic stability, the authors aimed to define the optimal Qp/Qs and clarify underlying physiological mechanisms in the immediate post-operative period.2
METHODS
Three neonates (all female; median age: 34 days [range: 30–41 days]; median weight: 2.6 kg [range: 2.5–2.7 kg]) who underwent the Norwood procedure were analysed (Figure 1). Patient-specific, 0–1-dimensional lumped parameter CFD models3 were generated using radial artery pressure waveforms, O2 saturation, and lactate measurements. Models were reconstructed at 30 post-operative blood-draw time points by minimising the difference between observed and simulated waveforms with a gradient descent algorithm. Qp/Qs and associated haemodynamic parameters were then calculated at each time point. Correlations between lactate levels, elapsed time, and haemodynamic indices were examined.4
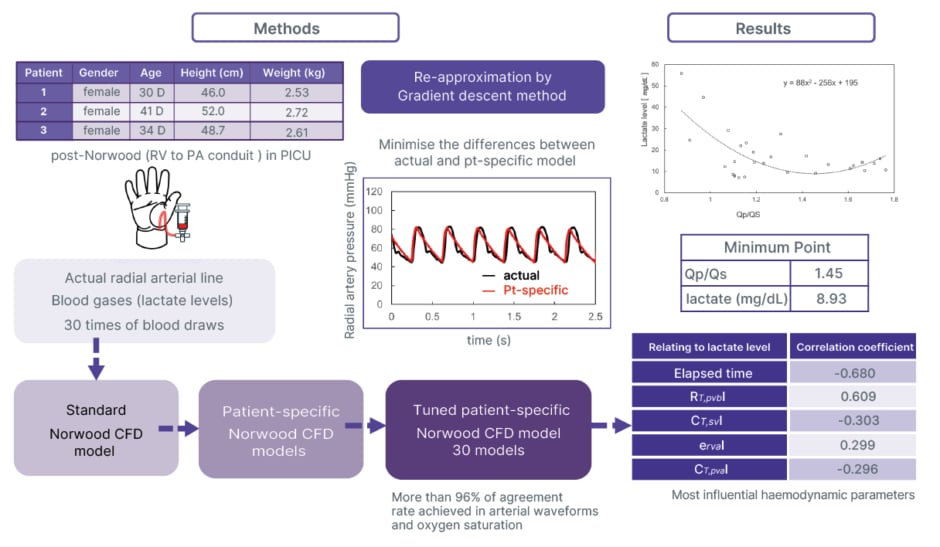
Figure 1: Flow of the research work.
CFD: computational fluid dynamics; CT,puaI: total pulmonary artery compliance index; CT,svI: total systemic venous compliance index; D: days; ervaI: right ventricle active elastance index; PA: pulmonary artery; PICU: paediatric ICU; pt: patient; Qp/Qs: pulmonary-to-systemic flow ratio; RT,pvbI: total pulmonary vascular resistance index; RV: right ventricle.
RESULTS
Model accuracy was high, with agreement rates of 96.0±2.5% for mean arterial pressure and 97.0±2.3% for arterial O2 saturation. Over time, pulmonary vascular resistance decreased (correlation coefficient [R]=–0.488) and pulmonary arterial compliance increased (R=0.350), reflecting natural post-operative adaptation. Importantly, declining lactate levels correlated with reduced pulmonary vascular resistance (R=0.609) and improved pulmonary arterial compliance (R=–0.296). Simultaneously, right ventricular active elastance decreased (R=0.299), indicating lower ventricular afterload and improved ventricular efficiency.
The lowest lactate concentrations coincided with a Qp/Qs of 1.45, not 1.0. This finding suggests that slightly higher pulmonary flow relative to systemic flow is associated with the most stable haemodynamics in the early post-operative Norwood circulation.
CONCLUSION
The authors successfully developed patient-specific CFD models capable of simulating Norwood haemodynamics by integrating routine physiological and biochemical data. These models provide a quantitative insight into dynamic cardiovascular adaptation after surgery, and reveal that lactate, a readily available biomarker, strongly reflects changes in vascular resistance, compliance, and ventricular function.
Most importantly, the authors’ findings showed that a ratio closer to 1.45 is associated with the most favourable balance between systemic and pulmonary circulations in the immediate post-operative period.
This study demonstrates the potential of patient-specific CFD modelling as a decision support tool in congenital heart surgery. By linking computational haemodynamic analysis with simple biological markers such as lactate, clinicians may refine post-operative management, individualise targets, and improve stability for vulnerable neonates after the Norwood procedure.







