BACKGROUND AND OBJECTIVES
Fibrotic interstitial lung disease (fibrotic ILD) entails a spectrum of different clinical entities characterised by an irreversible destruction of the alveolar wall, eventually leading to respiratory failure.1 Despite the development of antifibrotic treatments, overall survival rates remain low and good predictive and prognostic biomarkers are lacking.2 The aim of this prospective, multi-cohort study is to evaluate the value of the fibroblast activation protein (FAP) as a biomarker of fibrotic lung diseases with FAP inhibitor (FAPI) PET/CT as a minimally invasive imaging tool.3
MATERIALS AND METHODS
This is a prospective, exploratory, multi-cohort study including patients with fibrotic ILD and assigning them into separated cohorts depending on the underlying pathology and clinical evolution. An [18F]FAPI-74 or [68Ga]Ga-FAPI-46 PET/CT was performed at different time points depending on the patient cohort (Figure 1A and 1B). Quantitative PET parameters were compared before and during treatment. Finally, uptake values were compared to the clinical evolution of the patient in terms of pulmonary function tests. At follow-up, patients were classified as having progressive or non-progressive fibrotic ILD according to European Respiratory Society (ERS)/American Thoracic Society (ATS) criteria, and differences in baseline FAPI uptake parameters between the two groups were analysed using a two-sample independent t-test.4
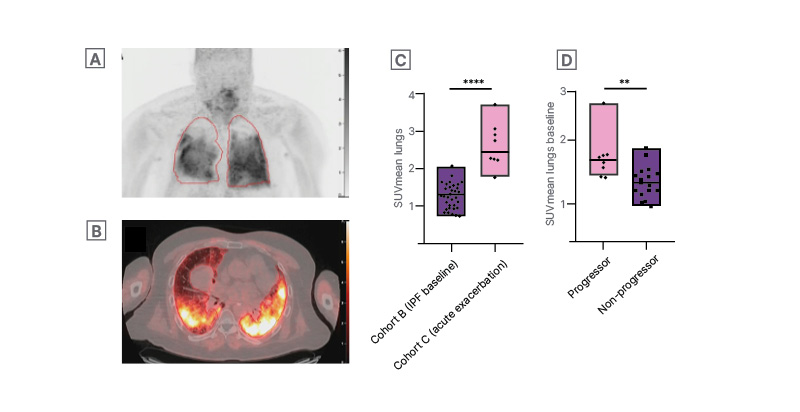
Figure 1: [68Ga]Ga-FAPI-46 PET/CT in patient with fibrotic interstitial lung disease with an acute exacerbation (A, B), and higher [68Ga]Ga-FAPI-46 uptake in patients with acute exacerbation (C) and those with progressive fibrotic disease phenotype (D).
MIP image (A) and corresponding axial section of the fused PET/CT images (B) of the [68Ga]Ga-FAPI-46 PET/CT in a fibrotic patient with ILD with an acute exacerbation of the disease. Two-sample independent t-tests showing a
significant higher SUVmean in patients suffering from an exacerbation compared to patients in basal conditions (p<0.0001) (C) as well as a significant higher baseline SUVmean in patients with a progressive phenotype compared to patients with a non-progressive phenotype of the disease (D).
FAPI: fibroblast activation protein inhibitor; ILD: interstitial lung disease; IPF: idiopathic pulmonary fibrosis;
MIP: maximum intensity projection; SUVmean: mean standardised uptake value.
RESULTS
At the time of the interim analysis, 31 patients underwent a baseline FAPI PET/CT, which was repeated after treatment initiation in 21 of these patients. A significant negative correlation was found between the mean standardised uptake value (SUVmean) and the forced vital capacity and diffusing capacity of the lungs at baseline (r=−0.55; p=0.001 and r=−0.64; p<0.0001, respectively). SUVmean values were significantly higher in patients suffering from an exacerbation of the disease compared to patients with stable disease (p<0.0001; Figure 1C). Moreover, a significant strong negative correlation was found between the change in SUVmean values and the change in diffusing capacity after 3 months of antifibrotic treatment (n=8; r=−0.87; p=0.005). Finally, a significant higher SUVmean at baseline was seen in patients with a progressive fibrotic ILD phenotype compared to the non-progressive phenotype (p=0.07; Figure 1D).
CONCLUSION
Preliminary results point out that FAPI PET/CT can be used as a minimally invasive imaging tool to monitor disease activity in different clinical entities of the fibrotic ILD spectrum. Further inclusion and follow-up of patients is ongoing to confirm the prognostic value of FAPI PET/CT in a larger patient cohort.







