Meeting Summary
This satellite symposium at the European Society for Medical Oncology (ESMO) Congress 2025, chaired by Rana McKay, Professor of Medicine and Urology at the University of California, San Diego, USA, explored evidence-based treatment strategies for metastatic castration-resistant prostate cancer (mCRPC) in the evolving treatment landscape. Bernard Tombal, Professor of Medicine at Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Brussels, Belgium, discussed combination treatment strategies with androgen receptor pathway inhibitors (ARPI) and radiopharmaceuticals. Pedro Barata, Medical Oncologist at the University Hospitals Seidman Cancer Center, Cleveland, Ohio, USA, addressed considerations for patients with homologous recombination repair (HRR) gene mutations, and the role of poly(ADP-ribose) polymerase inhibitors (PARPI) in this patient group. Kambiz Rahbar, Professor of Nuclear Medicine at University Hospital Münster, Germany, looked at emerging data on treatment sequencing in mCRPC following intensified treatment in the hormone-sensitive phase. The faculty illustrated how the data they presented inform treatment strategies by applying it to treatment decisions, for example, clinical cases.
Patients with Metastatic Castration-Resistant Prostate Cancer Today: Unmet Needs and Treatment Goals
Rana McKay
McKay opened this symposium by describing the natural history of mCRPC. Among patients experiencing disease recurrence after definitive treatment of localised prostate cancer, some may progress to metastatic disease while the cancer remains hormone sensitive (mHSPC) and later become castration resistant, while others may have rising prostate-specific antigen (PSA) while on androgen deprivation therapy (ADT), indicating castration resistance, before metastases develop. Both disease courses culminate in mCRPC, which McKay described as a ‘universally lethal’ disease state. Real-world median survival is only slightly longer than 2 years from the onset of mCRPC,1 although longer overall survival (OS) has been achieved in Phase III trials,2,3 suggesting a need to optimise use of the available treatment options to prolong the survival of patients with mCRPC. Recent data suggest that over 20% of patients developing mCRPC do not receive life-prolonging therapy, and of those receiving first-line treatment for mCRPC, only about half go on to receive second-line therapy, further diminishing in subsequent lines.1
There is therefore an unmet need to improve treatment and outcomes for patients with mCRPC. McKay summarised the goals of mCRPC treatment as prolonging survival, optimising safety, and preserving and improving quality of life, emphasising the importance of considering patients’ concerns and the goals that are most important to them, within a shared decision-making framework.
Evolving Landscape and New Options in Metastatic Castration-Resistant Prostate Cancer Treatment
Rana McKay, Bertrand Tombal
McKay gave an overview of the treatment landscape across clinical phases of prostate cancer. ADT is the backbone of prostate cancer treatment. As the treatment landscape evolves, ADT is increasingly integrated with a variety of other treatment options, including ARPIs, PARPIs, and radiopharmaceuticals. ARPIs have a prominent role across the disease continuum, and may be used in high-risk localised disease, as well as mHSPC and mCRPC.4 McKay highlighted treatment advances in the mHSPC setting in the last decade, with increasing recognition that intensified therapy with ARPIs improves progression-free survival when added to ADT, with or without taxane chemotherapy.5-10 She noted that this shapes the treatment history of patients entering the mCRPC setting, as increasing numbers of patients receive doublet or triplet therapy (ADT+ARPI±docetaxel) for mHSPC. However, recent data suggest that as many as 40% of patients with mHSPC progress to mCRPC without having received ARPI treatment.11,12
Tombal’s presentation focused on this patient group. For ARPI-naïve patients progressing on ADT, with or without docetaxel, the standard of care is an ARPI, with abiraterone or enzalutamide recommended as first-line options.13,14 Tombal posed the question of whether ARPI monotherapy is enough, and went on to discuss combination treatment approaches. PARPIs are indicated only for patients with HRR gene mutations; ARPI+PARPI combinations in this population were the subject of Barata’s presentation (below). Tombal discussed the role of radiopharmaceuticals in combination treatment with ARPIs, presenting recent clinical trial data that support this approach.
Radiopharmaceutical Plus Androgen Receptor Pathway Inhibitor Combination Therapy for Patients with Metastatic Castration-Resistant Prostate Cancer
Radium-223 is an α-emitting radionuclide which selectively targets metastases in the bone.15 Osteoblastic activity in bone metastases leads to high bone turnover; radium-223 is a calcium-mimetic and becomes incorporated into the bone matrix, where emission of α particles destroys both cancer cells and osteoblasts and osteoclasts. This disrupts a vicious cycle of positive feedback between osteoblasts and cancer cells, and makes the bone matrix ‘infertile soil’ for metastatic growth, thereby reducing the risk of bone complications and their negative impact on survival in prostate cancer.15
A significant survival benefit with radium-223 monotherapy was demonstrated in the ALSYMPCA trial,16 and supported by real-world OS findings in the REASSURE prospective observational study,17 which also confirmed a favourable long-term safety profile during 7 years’ follow-up.18 The Phase III EORTC-1333/PEACE-3 trial is an investigator-led academic trial, sponsored by the European Organisation for Research and Treatment of Cancer (EORTC), which assessed the effect of adding radium-223 to the ARPI enzalutamide in patients with mCRPC.2 Patients had ≥4 bone metastases but no known visceral metastases, and were asymptomatic or mildly symptomatic, with a World Health Organization performance status (WHO PS) of 0 or 1. All patients were receiving ADT; prior treatment with abiraterone and/or chemotherapy for mHSPC was permitted. In practice, very few enrolled patients had received abiraterone, while approximately 30% had received docetaxel. Patients were randomised to receive open-label treatment with enzalutamide 160 mg once daily, alone or with radium-223 55 kBq/kg every 4 weeks for six cycles. The primary endpoint was radiographic progression-free survival (rPFS); OS was a key secondary endpoint. A total of 446 patients were enrolled in PEACE-3, with a median age of 70 years in each treatment arm. The majority (88%) of patients randomised to radium-223 completed six cycles.2 Commenting on the high completion rate, Tombal recommended early use of radium-223 to allow completion of the treatment course to optimise efficacy, as completion of five or six cycles is associated with improved survival outcomes compared with fewer cycles.19,20
Addition of radium-223 to enzalutamide significantly increased median rPFS (19.4 versus 16.4 months; hazard ratio [HR]: 0.69; 95% CI: 0.54–0.87; p=0.0009; Figure 1A).2 At data cut-off for the final rPFS analysis, 80% of expected OS events had occurred. Interim OS analysis showed a significant OS benefit: median OS was extended by over 7 months, as of the data cut-off, with combination therapy versus enzalutamide alone (42.3 versus 35.0 months; HR: 0.69; 95% CI: 0.52–0.90; p=0.0031; Figure 1B).2 An Independent Data Monitoring Committee recommended continuation of the study to final OS analysis, with 100% of OS events to power the final analysis according to the statistical analysis plan, to confirm and further characterise results. Tombal updated the audience with news that the trial has reached its final OS endpoint, and EORTC has revealed that the final analysis reinforces the findings of the interim analysis, confirming that the addition of radium-223 to enzalutamide significantly prolonged OS (unpublished data). Time to next systemic treatment was also significantly delayed by addition of radium-223 to enzalutamide: at 24 months’ follow-up, over 50% of patients randomised to enzalutamide alone had started their next line of therapy, while only 30% of patients on enzalutamide plus radium-223 had started further therapy, approximately 18 months after completing six cycles of radium-223.2 PSA data further supported a synergistic effect of radium-223 combined with enzalutamide. A significantly higher proportion of patients achieved a PSA response (≥90% decline) in the combination arm compared with the enzalutamide monotherapy arm (51% versus 34% at 6 months; 55% versus 38% at 12 months), with significantly shorter time to achieving confirmed PSA response.21 Safety data showed limited additional toxicity associated with adding radium-223 to enzalutamide. A slight increase in Grade 3/4 drug-related adverse events was observed (28% versus 19% of patients), but no individual adverse event increased in incidence by more than 5%.2
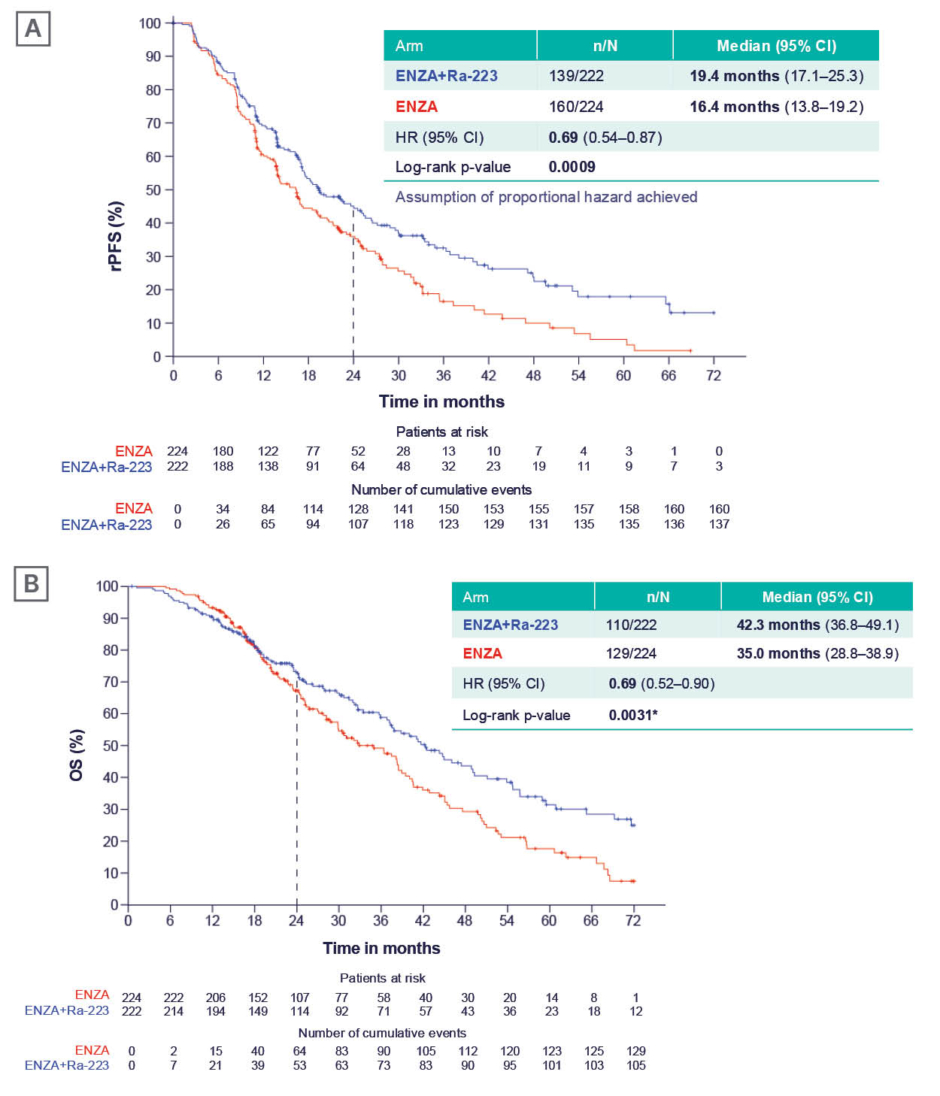
Figure 1: Efficacy of enzalutamide+tadium-223 versus enzalutamide monotherapy in the PEACE-III trial.2
*Pre-set level of significance for interim analysis was ≤0.0034.
A) rPFS (primary endpoint); and B) OS (interim analysis).
rPFS and OS were analysed using a Cox proportional hazards survival model stratified by baseline pain score, prior docetaxel, and BPA use at randomisation. The assumption of proportional hazards was not met for OS, and restricted mean survival time sensitivity analyses did not give unequivocal significance. Therefore, the study has continued to final OS analysis.
BPA: bone-protecting agent; ENZA: enzalutamide; HR: hazard ratio; OS: overall survival; Ra-223: radium-223; rPFS: radiographic progression-free survival.
Tombal also presented efficacy data from a Phase II trial of the radioligand lutetium-177 prostate-specific membrane antigen-617 (177Lu-PSMA-617), in combination with enzalutamide. rPFS and OS were prolonged in patients with mCRPC receiving enzalutamide plus 177Lu-PSMA-617 (n=83) compared with enzalutamide alone (n=79),22 warranting validation of this regimen in a Phase III trial.
Protecting Bone Health in Patients with Metastatic Castration-Resistant Prostate Cancer
Tombal emphasised the importance of administering a bone-protecting agent (BPA) to patients undergoing treatment for mCRPC. Patients with bone metastases are at particularly high risk of skeletal-related events, including fractures, and guidelines recommend preventive administration of BPAs such as zoledronic acid or denosumab,4,13,14 although Tombal noted that adherence to this recommendation is suboptimal in real-world practice. During the PEACE-3 trial, a protocol amendment mandated the use of BPAs. Approaches to BPA use varied between study centres before it was mandated for all patients. Among approximately 120 patients enrolled before the protocol amendment, approximately half received preventive BPA before/during study treatment, while the other half received no BPA, or started BPA only after a fracture. An exploratory analysis in these patient subgroups showed that, as well as decreasing fracture rate,23 BPAs appeared to enhance the efficacy of the main study treatments, with considerably longer rPFS and OS in patients who were taking BPAs than those who were not.24 This hypothesis-generating post-hoc analysis suggests a synergistic effect beyond bone protection.
Clinical case challenge #1: Androgen receptor pathway inhibitor- and docetaxel-naïve patient
Throughout the symposium, speakers contextualised the data they presented by applying it to treatment decisions for an example patient, with clinical characteristics reflecting the different settings they discussed. McKay introduced their model patient as a 65-year-old male, a retired teacher with a history of hypertension.
In the first scenario, the patient was treatment naïve, having presented to his primary care physician in January 2016 for routine health maintenance, where screening revealed PSA of 20 ng/mL. MRI of the prostate showed locally extensive T3a disease, and CT of the chest, abdomen, and pelvis showed pelvic and retroperitoneal nodes, and an isolated bone metastasis in the iliac bone, confirmed on bone scan. Prostate biopsy confirmed prostatic adenocarcinoma with Gleason score 4+3=7. He started ADT in February 2016, and received an external beam radiotherapy to the primary cancer and nodes. He achieved a PSA nadir <0.01 ng/mL after 3 months and remained on ADT for 3 years, but discontinued in February 2019 due to toxicity. The patient was monitored, and by July 2022, his PSA level had risen to 10 ng/mL, and the disease had progressed to T3b (locally invasive into the seminal vesicle) on MRI. No metastases were present on CT or bone scan. ADT monotherapy was restarted in August 2022; PSA returned to <0.01 ng/mL after 2 months, and was controlled for approximately 2 years. However, in November 2024, PSA was rising, and by July 2025, PSA was 14 ng/mL, with a doubling time of 2 months. CT revealed pelvic and retroperitoneal nodes and metastases in the pelvis, lumbar spine, and ribs, confirmed on bone scan. Tumour somatic gene profiling showed no HRR mutations. He remained asymptomatic, with an Eastern Cooperative Oncology Group performance score (ECOG PS) of 0.
After presenting her overview of the mCRPC treatment landscape, McKay asked the audience how they would treat this patient on emergence of metastatic disease. The majority (53%) selected ARPI monotherapy.
Tombal repeated the question, considering the same patient case, at the end of his presentation. After seeing the PEACE-3 data, the majority of respondents (84%) selected enzalutamide+radium-223.
Treating Metastatic Castration-Resistant Prostate Cancer in Patients with Homologous Recombination Repair Gene Mutations
Pedro Barata
HRR-related gene mutations are prevalent in patients with mCRPC (approximately 25% of patients)25 and are associated with worse prognosis than HRR mutation (HRRm)-negative status.25-27 Genetic testing is underused, with less than 40% of patients in Europe undergoing testing.28
The efficacy and safety of various ARPI+PARPI combinations have been investigated in clinical trials,29-35 including the Phase III TALAPRO-2 trial, which enrolled over 1,000 patients, including a cohort with various HRR mutations.35 Significant improvements in rPFS and OS were seen in HRRm-positive (HRRm+) patients receiving talazoparib plus enzalutamide compared with those on enzalutamide alone; median OS was extended by 14.0 months (45.1 versus 31.1 months; HR: 0.62; 95% CI: 0.48–0.81; p<0.0005).3 Barata remarked that this represents meaningful benefit to patients, highlighting the value of genetic testing to identify HRRm+ patients and give them the chance to benefit from ARPI+PARPI treatment. However, toxicities must be managed to keep patients on treatment. The combination regimen was associated with increased incidence of haematological adverse events, most commonly anaemia, which typically occurred in the first 3–4 months of treatment.3 Barata discussed how trial investigators had learned to manage anaemia, with strategies including dose adjustments and blood transfusions, and suggested that there would be a learning curve for urologists who lack experience with PARPIs.
ARPI+PARPI regimens that have demonstrated efficacy in mCRPC are now also being investigated in the hormone-sensitive setting. The AMPLITUDE trial investigated nariparib plus abiraterone in patients with mHSPC and ≥1 HRR mutation. Barata showed results for a subgroup of patients with BRCA1/2 alterations, in whom addition of nariparib to standard-of-care ARPI significantly improved rPFS.36 However, benefits of combination treatment must be balanced against increased toxicity. Barata commented that it will be important to see more safety data, with longer treatment exposure and follow-up, as PARPIs move into earlier disease settings.
Radiopharmaceutical+PARPI Combination Therapy for Patients with HRR Mutation-Positive Metastatic Castration-Resistant Prostate Cancer
The Phase II COMRADE trial investigated the efficacy of radium-223 in combination with olaparib in patients with mCRPC. Although the HRRm+ subgroup was small (n=23), clinical benefit was observed in this cohort, with median rPFS extended from 4.7 to 5.5 months (HR: 0.47; 90% CI: 0.22–1.01) when radium-223 was added to olaparib.37
The combination of olaparib with 177Lu-PSMA-617 has also shown promising results in a Phase I study.38 Efficacy signals in these studies provide proof-of-concept for combining radiopharmaceuticals and PARPIs, warranting further investigation to explore the balance of efficacy and safety.
Clinical case challenge #2: Homologous recombination repair mutation-positive patient
Barata revisited the patient case described earlier by McKay, describing a slightly different scenario. In this case, the patient followed the same clinical course, with progression on ADT, but genetic testing on progression revealed a BRCA2 mutation (the most common HRR gene alteration found in mCRPC).35
Based on the clinical trial data Barata presented, ARPI+PARPI combination therapy would be the preferred treatment option for this patient. Discussing sequencing of ARPIs and PARPIs in ARPI-naïve patients, he advocated early use of combination therapy as a rational approach to optimising suppression of androgen signalling pathways. A treatment sequence that does not use the most effective treatment option first, Barata commented, risks losing the patient to follow-up before they move on to second-line therapy.
The combination of a PARPI with a radiopharmaceutical agent remains an investigational approach in the HRR+ mCRPC setting at present, but Phase I/II data suggest this could be a promising approach in the future.
Sequencing of Treatments for Metastatic Castration-Resistant Prostate Cancer
Rana McKay, Kambiz Rahbar
The data on ARPI combinations presented by Tombal and Barata were from trials that included predominantly ARPI-naïve patients. However, McKay reiterated, ARPIs are indicated for mHSPC and non-metastatic CRPC, and increasing numbers of patients developing mCRPC will have received prior ARPI treatment. For patients progressing to mCRPC on an ARPI, several studies suggest that switching to a different ARPI provides limited benefit.29,30,39-42 Adding docetaxel can be beneficial for taxane-naïve patients,43,44 but for those who have received prior docetaxel, rechallenge is minimally effective.45,46 Alternative taxane chemotherapy with cabazitaxel can be considered post-docetaxel and ARPI.42 However, other treatment options also have a place in the management of pre-treated patients who are not candidates for PARPIs.
Role of Radiopharmaceuticals in Pre-treated Patients with Metastatic Castration-Resistant Prostate Cancer
Radiopharmaceuticals are indicated for second/third-line treatment following ARPIs and docetaxel for mCRPC,4,13,14 but may also have a role in earlier lines for patients with mCRPC who have already received ARPIs and/or docetaxel in the mHSPC setting. Two radiopharmaceutical products are approved for mCRPC: radium-223 for patients with bone metastases and no visceral metastases, and 177Lu-PSMA-617 for those with ≥1 PSMA-positive metastasis at any site. These products differ in terms of both type of radiation and targeted delivery of that radiation; Rahbar outlined their respective properties. Radium-223, as Tombal explained earlier, is an α-emitting radionuclide.15 α radiation has high energy but short range; radium-223 induces double-stranded DNA breaks that are difficult for tumour cells to repair,15 but has limited penetration. Cytotoxic effects are therefore localised to sites of uptake in bone metastases, limiting damage to healthy tissues.15 177Lu-PSMA-617 is a radioligand that delivers β-particle radiation to PSMA-expressing tissues,47 including PSMA-positive metastases in bone and other organs. β radiation has lower energy, inducing single-stranded DNA breaks, but greater penetration than α radiation. Due to the penetrative nature of the radiation delivered by 177Lu-PSMA-617, patients are advised to avoid close contact with other people, including sleeping separately from partners, for up to 15 days after each administration (specific recommendations vary between countries). Both are administered over six cycles, radium-223 with a body weight-adapted dosage of 55 kBq/kg every 4 weeks, and 177Lu-PSMA-617 at a fixed dose of 7,400 MBq every 6 weeks. Antiemetics are recommended prior to the administration of 177Lu-PSMA-617.
Given their differing and complementary mechanisms of action, sequencing of radiopharmaceuticals is a rational approach. RaLu was a retrospective, multicentre study evaluating outcomes with 177Lu-PSMA-617 in approximately 200 patients who had received ≥1 cycle of radium-223 (Figure 2).48,49 Median OS from the start of 177Lu-PSMA-617 was 12 months (Figure 2),49 which is in line with published values for OS with 177Lu-PSMA-617, including in the VISION trial.47 Median OS from the start of radium-223 treatment was 33 months (Figure 2).49 No increase in toxicity was observed when 177Lu-PSMA-617 was given after radium-223, compared with its established safety profile, supporting the feasibility of this sequencing approach.48,49 The reverse sequence has been investigated in a small retrospective cohort study, LuRa (n=19), which suggested that radium-223 can be given after 177Lu-PSMA-617.50
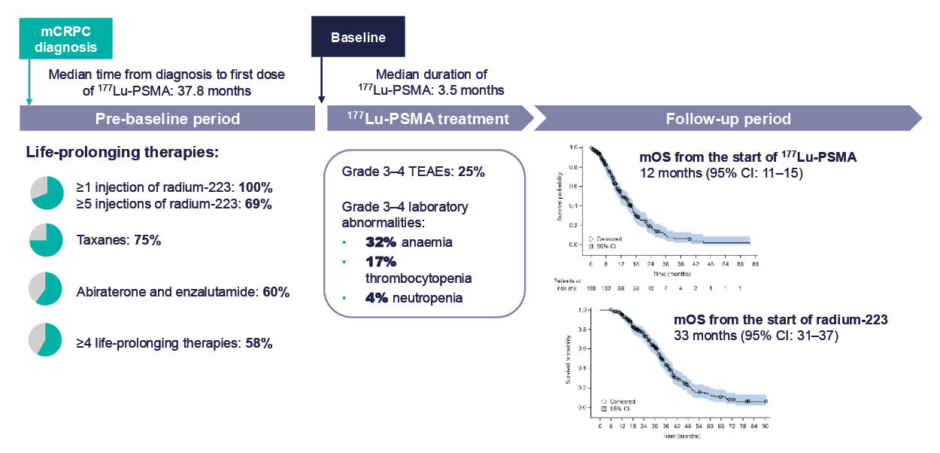
Figure 2: Overall survival and safety in patients receiving sequential treatment with radium-223 and 177Lu-PMSA in the RaLu study.49
mCRPC: metastatic castrate-resistant prostate cancer; mOS: median overall survival; PMSA: prostate membrane specific androgen; TEAE: treatment-emergent adverse event.
Rahbar went on to describe the RADIANT trial (NCT04597125),51 an ongoing Phase IV study investigating the efficacy and safety of radium-223 in early lines of therapy in mCRPC. The study population comprises patients with mCRPC, with ≥2 bone metastases and no visceral metastases, who have progressed on or after one line of ARPI for an approved prostate cancer indication (mHSPC or mCRPC) plus taxane chemotherapy unless contraindicated or refused. A range of treatment histories are represented in the trial population (Figure 3) to provide data on radium-223 sequencing in the contemporary mCRPC landscape. Over 600 patients have been randomised to receive either six cycles of radium-223 or a second ARPI (different to their first-line ARPI). The primary endpoint is OS, and results are expected in 2026.51
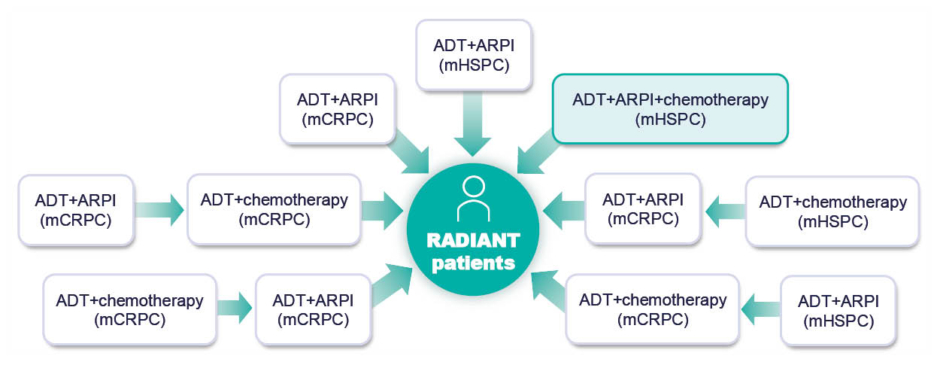
Figure 3: Prior treatments for metastatic hormone-sensitive prostate cancer and/or metastatic castrate-resistant prostate cancer in patients enrolling in the RADIANT trial.
ADT: androgen deprivation therapy; ARPI: androgen receptor pathway inhibitor; mCRPC: metastatic castration-resistant prostate cancer; mHSPC: metastatic hormone-sensitive prostate cancer.
Clinical case challenge #3: Pre-treated patient
The third clinical case challenge featured a patient with a history of prior ADT+ARPI+docetaxel triplet therapy for mHSPC. McKay reframed the example patient’s history, a 65-year-old male with no other significant medical history, but with a more substantial disease on diagnosis of prostate cancer than previous examples.
The patient had a high PSA level of 99 ng/mL on initial presentation, and Stage T3a prostate cancer was detected on MRI. CT revealed pelvic and retroperitoneal nodes. Lung metastasis and multiple bone metastases were also detected and confirmed by bone scan and lung biopsy. In this scenario, it is appropriate to intensify therapy in the mHSPC setting; first-line treatment was darolutamide and docetaxel in addition to ADT, initiated in August 2022.
The patient did well on triplet therapy and achieved a PSA nadir <0.01 ng/mL after 2 months. Two years later (late 2024), PSA was rising, but lung metastases had resolved, and bone metastases were stable. However, new and increased bone metastases were found in the spine, pelvis, and ribs in July 2025. Lymph node metastases had also developed, but no visceral metastases were detected. PET-CT was performed and showed that lesions were PMSA-negative.
Rahbar considered treatment options for this patient, asking the audience to vote on their preferred treatment choice for first-line treatment of mCRPC following prior triplet therapy for mHSPC. Half indicated that they would select a second taxane chemotherapy regimen, and half opted for radiopharmaceuticals (predominantly radium-223, given this patient’s metastatic profile of PMSA-negative bone metastases and absence of visceral metastases).
Conclusions and Future Perspectives
The symposium concluded with a panel discussion. The speakers concurred that ADT+ARPI+chemotherapy triple therapy sets a high bar for efficacy and is likely to be standard-of-care for patients requiring escalation of therapy for mHSPC for the foreseeable future. In the rapidly evolving mCRPC landscape, research to understand where different treatment options sit in the treatment paradigm is important. The evidence presented in this symposium supports the use of combination therapy with ARPIs+radiopharmaceuticals early in the disease course in mCRPC, including asymptomatic disease. An audience poll indicated that, after seeing the PEACE-3 data presented in the symposium, most (>60%) saw a future role for radium-223 plus enzalutamide as first-line therapy for mCRPC. ARPI+PARPI, and potentially radiopharmaceutical+PARPI, combinations have a role in the HRRm+ portion of the mCRPC population. Alongside anti-cancer agents, BPAs are a critical element of the management of mCRPC, to mitigate risk of fractures.
Data were also shown that support the approach of sequencing radiopharmaceuticals. Tombal noted that it is not a case of choosing between radium-223 and 177Lu-PMSA-617, but rather determining the position of each in the treatment sequence. This will depend on patient characteristics including metastatic sites and biomarkers, but Tombal stated that, in his view, many patients could benefit from early treatment with radium-223, while metastases are confined to the bones, followed by 177Lu-PMSA-617.







