Meeting Summary
Anal and colorectal cancers are complex gastrointestinal malignancies that continue to pose therapeutic and diagnostic challenges worldwide. This article is based on the Industry Satellite Symposium, ‘Exploring New Horizons and Emerging Topics in Squamous Anal Cancer and CRC’, delivered by four world-renowned experts during the European Society for Medical Oncology (ESMO) 2025 Annual Congress through a series of informative talks, case studies, and an interactive panel discussion. Christelle de la Fouchardière, Medical Oncologist at Paoli-Calmettes Institute, Marseille, France, opened the meeting with an introduction to squamous cell anal cancer (SCAC), describing its epidemiology, aetiology, and diagnosis, as well as strategies for prevention. Gunnar Folprecht, Professor and Medical Oncologist at the University Hospital Carl Gustav Carus, Dresden, Germany, then described multidisciplinary approaches to early-stage SCAC. This was followed by a presentation from Dominik Modest, Professor and Medical Oncologist at the Charité Universitätsmedizin, Berlin, Germany, who discussed current treatment strategies for advanced SCAC. The final two presentations were given by Sheela Rao, Consultant Medical Oncologist at the Royal Marsden Hospital, London, UK, who was also chairing the meeting. Rao first described the evolving landscape in advanced anal cancer and concluded by discussing present and future targets in colorectal cancer (CRC), with a focus on KRAS inhibition and transforming growth factor beta (TGF-β).
Squamous Anal Cancer: A Rare Human Papillomavirus-Driven Tumour where Early Diagnosis is Key
Introduction to Squamous Anal Cancer
de la Fouchardière began her presentation by explaining that, although SCAC accounts for the majority of anal cancer cases, it remains an uncommon malignancy, with an estimated 55,000 new diagnoses and 22,000 deaths worldwide in 2020.1,2 SCAC occurs more frequently in women, particularly those over 50 years of age, but an increasing incidence among younger men has been observed.3 Data from population-based registries in the UK and the USA indicate a 2–3% annual rise in incidence,4,5 with increases in incidence and mortality predicted worldwide to 2045.6
Human papillomavirus (HPV) infection is the principal risk factor for SCAC, being identified in approximately 90% of cases, most commonly with genotypes 16 and 18.7 Other risk factors include immunosuppression, particularly in individuals with HIV infection or recipients of solid organ transplants, as well as a history of long-term steroid use.8,9
HPV is a small, non-enveloped DNA virus comprising an 8-kilobase circular genome, with early (E) and late (L) genes encoding the viral proteins.10 Among over 180 HPV genotypes, 12 are classified as oncogenic, of which types 16 and 18 are most frequently implicated in SCAC.10,11 The virus targets proliferating basal cells in the squamous epithelium,10,11 then stable integration of the HPV genomic element into the host genome and subsequent expression induces premalignant lesions, which may progress to cancer.8
HPV infection is widespread, with a peak incidence around the age of 25 years.12 Most infections are asymptomatic and clear spontaneously within 2 years; however, persistent infection with oncogenic subtypes may progress to high-grade lesions and invasive cancer.12,13 de la Fouchardière explained that HPV positivity is also an important prognostic factor in SCAC. HPV-positive tumours typically exhibit improved responses to chemoradiotherapy, with lower recurrence rates and superior overall survival compared with HPV-negative tumours.14 Conversely, adverse prognostic features include ulceration, lymph node involvement, and male sex.15
Clinical Presentation and Diagnostic Workup
According to the ESMO 2021 clinical practice guidelines, diagnosis of SCAC should begin with digital rectal examination followed by biopsy for histopathological confirmation and p16 immunohistochemistry, with additional HPV testing when indicated.16 Pelvic MRI and PET-CT with [18F]2-fluoro-2-deoxy-D-glucose are recommended for staging, providing superior accuracy for local and nodal assessment.16
Most patients with anal cancer present with localised or locoregional disease,17 with diagnosis at an earlier clinical stage strongly correlating with improved overall survival (OS).18 This highlights the importance of early detection and provides a rationale for targeted screening. High-risk populations include men who have sex with men, individuals with HIV, women with prior HPV-related gynaecological cancer, and immunosuppressed individuals.19
HPV vaccination represents a cornerstone of primary prevention, substantially reducing the incidence of HPV infection and associated malignancies.20 Current immunisation programmes target both girls and boys aged 9–14 years, with catch-up schedules for young adults,21 although global vaccination coverage remains suboptimal at only 40–55%.20
de la Fouchardière concluded that SCAC is a rare but increasingly prevalent malignancy in which HPV infection acts as the primary tumour-initiating event.6,7 Anal cancer should be diagnosed in line with local guidelines and should include histological confirmation of SCAC,16 with a timely diagnosis essential to improve OS.18 de la Fouchardière noted that although HPV vaccination programmes have the potential to significantly reduce the burden of HPV-related malignancies such as SCAC,20 vaccination rates among high-risk groups remain suboptimal, even in high-income countries, underscoring the need for continued public health efforts.20
Navigating Early-Stage Squamous Anal Cancer: Multidisciplinary Treatment Approaches
In his presentation, Folprecht described current treatment approaches for patients with early-stage SCAC, drawing on the case of a female in her late 60s presenting with well-differentiated, p16-positive squamous cell carcinoma as an illustrative example.
The patient underwent a comprehensive diagnostic workup, including pelvic MRI and fluorodeoxyglucose PET, which clearly delineated the primary lesion, confirmed the absence of metabolic activity in regional lymph nodes, and excluded adrenal or distant metastases. Imaging demonstrated a tumour of approximately 1 cm in diameter with a cranio-caudal length of 4 cm, without nodal involvement. The patient’s medical history included smoking-related emphysema, HIV serology was negative, and dihydropyrimidine dehydrogenase function was normal.
Based on the tumour, node, and metastasis classification, the lesion was categorised as Stage IIA (T2N0M0), representing a localised, yet not early microinvasive disease.18,22 Early-stage/localised anal cancer is considered to fall within the range of Stage I to IIIa.18,22 Folprecht explained that the only surgical option for the patient would be abdominal perineal resection with a permanent stoma, which was ruled out in preference to combined radiotherapy and chemotherapy with mitomycin C (MMC) or cisplatin, as recommended by both the American Society of Clinical Oncology (ASCO) and ESMO guidelines.16,23
Evidence from the UK Collaborative for Cancer Clinical Research (UKCCCR) ACT I trial showed that the addition of 5-fluorouracil (5-FU) and MMC to radiotherapy among 577 patients with squamous, basaloid, or cloacogenic cell carcinoma of the anal canal/margin resulted in a substantially reduced risk of death from anal cancer.24
Questions regarding the role of induction or maintenance chemotherapy have been addressed in subsequent investigations. Induction therapy prior to chemoradiation did not improve disease-free survival in the RTOG 9811 trial, and maintenance therapy after definitive treatment failed to demonstrate progression-free benefit in ACT II.25,26 Therefore, concurrent fluoropyrimidine plus MMC with radiotherapy remains the standard of care for early-stage/localised anal cancer.16 In current chemotherapy protocols, 5-FU is administered either by continuous infusion during Weeks 1 and 5, or alternatively, oral capecitabine, combined with MMC, is given once or twice throughout treatment. Radiotherapy dosing typically ranges from 50 Gy or more for advanced lesions, with lower doses occasionally used for smaller T1–T2 tumours. In the present case, the patient received chemoradiation with MMC, including nodal basins within the radiation field.
Prognosis after definitive chemoradiation depends primarily on tumour size and nodal status. Patients with T2N0 tumours can expect a 5-year progression-free survival (PFS) of approximately 70% and a high probability of cure.27 However, some patients do not achieve complete response and require salvage surgery. Post-treatment analyses have shown that early surgical assessment, within 11 weeks after chemoradiation, may lead to overtreatment, as some tumours continue to regress beyond this period. Re-evaluation at approximately 26 weeks allows more accurate distinction between residual disease and delayed response (Figure 1).28
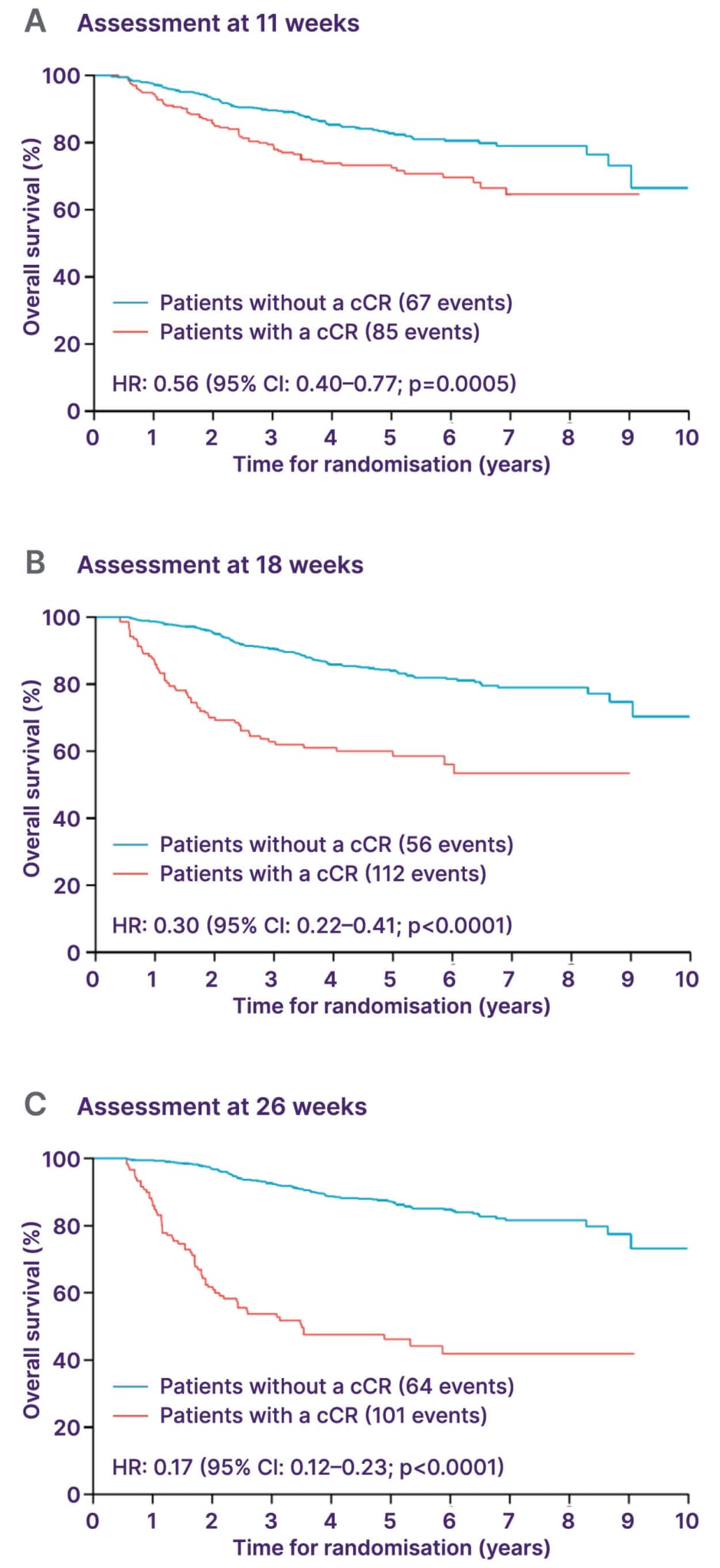
Figure 1: ACT-2 post-hoc analysis.28
Adapted from Glynne-Jones R et al.28 and licensed under CC BY 4.0.
cCR: complete clinical response; HR: hazard ratio.
Surgery remains an important option for selected patients. For tumours of the anal margin confined outside the canal, local excision preserving the sphincter may be feasible, provided a histologic margin of at least 1 mm is achieved. MRI assists in assessing suitability for conservative surgery.16,29 Nevertheless, for most anal canal lesions, sphincter-preserving surgery is rarely possible.16,29
Salvage abdominoperineal resection is indicated for persistent or recurrent disease, but is associated with substantial morbidity and the need for permanent stoma.16 Despite these limitations, a proportion of patients undergoing salvage surgery achieve durable remission, underscoring the importance of multidisciplinary management and careful patient selection.29
In conclusion, Folprecht noted that only a small subset of anal cancers are responsive to primary surgery, and that most of these are early anal margin cancers (cT1N0M0).16 Based on current guidelines, early-stage/localised disease should be treated with chemoradiotherapy (fluoropyrimidine+MMC+radiotherapy) in the first-line setting,16 while induction or maintenance therapies do not appear to result in survival benefits for patients.25,26 In addition, salvage surgery should be considered in patients with residual or locally recurrent disease, although this is a significant procedure, requiring lifelong management.16 Folprecht also remarked that a multidisciplinary approach is mandatory, involving radiation oncologists, medical oncologists, surgeons, radiologists, and pathologists.16
Advanced Squamous Anal Cancer: Current Treatment Strategies
Systematic Assessment of Chemotherapy Regimens
Modest began by noting that management of advanced or metastatic SCAC has evolved significantly over recent years, guided by both pragmatic clinical experience and emerging evidence from collaborative trials. Two major areas of development have shaped current practice: the optimisation of chemotherapy backbones for unresectable disease and the integration of newer, less toxic regimens for improved survival and tolerability.
Historically, treatment of metastatic SCAC relied on small observational studies and institutional experiences to direct therapeutic choices, where combinations of platinum compounds with fluoropyrimidines were commonly employed.30 MMC, long used in concurrent chemoradiation, was also included in early metastatic regimens, though its use has since declined. Second-line approaches were inconsistent, often involving taxanes such as paclitaxel or irinotecan after progression on platinum-based therapy.30 Reported PFS ranged from 6–9 months, with OS rarely exceeding 12–15 months, underscoring the urgent need for more standardised treatment strategies.30
The pivotal InterAAct trial marked a major step forward by establishing carboplatin and paclitaxel as a modern first-line standard of care. This international randomised Phase II study compared carboplatin–paclitaxel with cisplatin–5-fluorouracil and demonstrated comparable response rates, but a more favourable safety profile for the carboplatin–paclitaxel regimen.31 Although PFS was similar between arms, OS was favoured by carboplatin–paclitaxel, reaching a median of approximately 20 months, which is considered a meaningful improvement for this rare malignancy.31 The regimen’s tolerability and feasibility in a global multicentre setting solidified its adoption as the preferred first-line backbone in current guidelines.
The choice of carboplatin–paclitaxel has since become the foundation for ongoing research and combination studies. Its balanced efficacy and safety make it suitable for a broad range of patients, including those with comorbidities or reduced performance status.16 For subsequent therapy after platinum failure, treatment options remain heterogeneous. Irinotecan-based regimens are commonly used, although no second-line standard has been firmly established. Immunotherapy with programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors has emerged as a promising approach for this group, offering durable responses in selected patients with previously treated metastatic disease.16
Parallel efforts to intensify systemic therapy have produced encouraging results. The French Epitopes-HPV02 trial investigated a triplet regimen combining docetaxel, cisplatin, and 5-FU, administered either every 3 weeks or in a modified biweekly schedule.32 The study achieved high response rates and prolonged PFS compared with historical doublet regimens. Notably, the biweekly modification substantially reduced the incidence of severe haematological and gastrointestinal toxicities, making it more manageable for clinical practice.32 Although triplet therapy may not be suitable for frail or comorbid patients, it represents a valuable option for those requiring rapid tumour control, particularly when disease burden is high.
Across studies, the consistency of clinical outcomes supports a growing optimism in this challenging field.31-34 Median OS for patients with advanced or metastatic SCAC now exceeds 20 months, with some regimens achieving response rates above 70%. These gains reflect both improved systemic therapies and the benefits of structured multidisciplinary collaboration in managing a rare and complex cancer type.
From a molecular perspective, therapeutic innovation remains limited by the relatively simple genomic landscape of SCAC.35 Actionable mutations are uncommon, and current molecular profiling has yet to identify consistent targets amenable to precision therapies.35 While exploratory work is ongoing into PI3K inhibitors and poly(ADP-ribose) polymerase-targeted agents, the heavy prior exposure to platinum chemotherapy complicates their potential benefit.35 As such, future progress is expected to come primarily from immunotherapy and rationally designed combination approaches rather than from highly specific molecular targeting.
Modest concluded by emphasising that carboplatin–paclitaxel remains the established standard of care for patients with locally advanced, unresectable, or metastatic SCAC based on current evidence.31 The modified docetaxel, cisplatin, and 5-FU regimen has shown promising efficacy and may represent a viable alternative chemotherapy backbone.32 Ongoing advances in combined checkpoint inhibition further reinforce the role of carboplatin–paclitaxel as the foundation of emerging standard-of-care strategies in this setting.34
Immunotherapy and Beyond: The Evolving Treatment Landscape in Advanced Anal Cancer
Rao opened her presentation by noting that, as an HPV-driven malignancy, SCAC displays viral antigens that elicit chronic immune activation, leading to T cell exhaustion within the tumour microenvironment.36 This biological profile provides a strong rationale for PD-1 and PD-L1 blockade, which can restore antitumour immune activity and modify disease trajectory.
For patients with advanced or metastatic disease, a combination of carboplatin and paclitaxel remains the standard first-line therapy.16 Historically, second-line regimens relied on repurposed chemotherapy combinations with limited benefit, highlighting the need for novel approaches.16 The recent FDA approval of the PD-1 inhibitor, retifanlimab, represents a pivotal development, with retifanlimab becoming the first immunotherapy licensed in the USA specifically for advanced anal cancer.37
Early single-agent immunotherapy studies established proof of concept. Nivolumab, pembrolizumab, and retifanlimab each demonstrated activity in previously treated patients, producing response rates between 11–24%, with median OS around 10–12 months.38-40 Rao noted that although response rates were modest, these studies help to establish immunotherapy as a clinically meaningful option and pave the way for combination regimens.
Immuno-oncology in Combination with Chemotherapy in First-Line Advanced Anal Cancer
The Phase II SCARCE-PRODIGE 60 trial evaluated atezolizumab with the modified docetaxel, cisplatin, and 5-FU regimen in untreated advanced SCAC.33 The study did not improve PFS compared with chemotherapy alone, but Rao observed that exploratory analysis hinted that patients with higher PD-L1 expression might derive greater benefit. These findings reinforce the rationale for combining immune checkpoint inhibitors with chemotherapy.
More conclusive evidence came from the Phase III POD1UM-303/InterAAct 2 trial, which investigated carboplatin–paclitaxel with or without retifanlimab in the first-line setting in 308 patients with locally recurrent or metastatic SCAC (Figure 2).34,41 The study met its primary endpoint, demonstrating improved PFS (9.3 months with retifanlimab versus 7.4 months with chemotherapy alone; p=0.0006).34 Early separation of the survival curves suggested a sustained treatment effect, with median OS extending to 29 months in the immunotherapy arm (Figure 3).34,41 The addition of retifanlimab nearly doubled the median duration of response from approximately 7 to 14 months and increased the overall response rate to approximately 56%. Toxicities were consistent with prior experience, largely immune-related but manageable, without compromising chemotherapy delivery.34,41
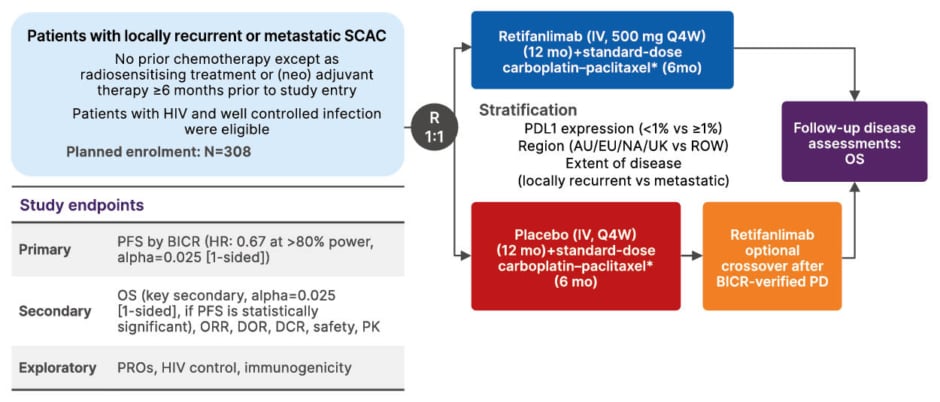
Figure 2: POD1UM-303/InterAACT 2: study design.34,41,42
*Standard-dose carboplatin–paclitaxel. Carboplatin: area under the curve 5 mg/mL on Day 1; Paclitaxel: 80 mg/m2 IV on days 1, 8, and 15. Each cycle=28 days. 6 months/24 weeks (6 cycles).
AUC: area under the curve; BICR: blinded independent central review; DCR: disease control rate; DOE: duration of response; HR: hazard ratio; IV: intravenous; mo: months; NA: North America; PD: progressive disease; PK: pharmacokinetics; PRO: patient-reported outcome; Q4W: every 4 weeks; ROW: rest of the world; SCAC: squamous cell anal cancer; vs: versus.
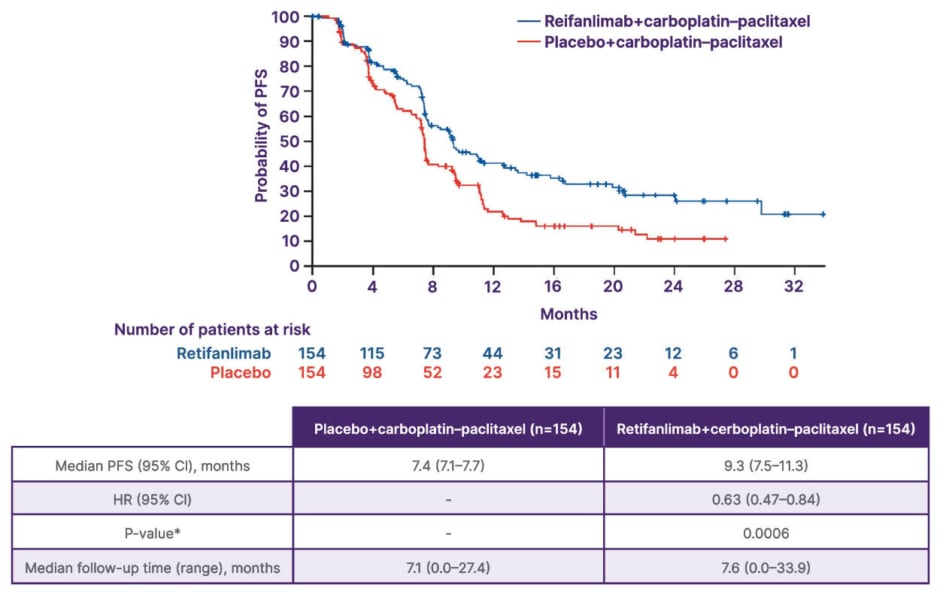
Figure 3: POD1UM-303/InterAACT 2: PFS by BICR (primary endpoint).34
*Stratified log-rank test with a one-sided significance level of 2.5%. Stratification factors: region of the world, extent of disease, and PD-L1 expression status.
Adapted from Rao S et al.34 and licensed under CC BY 4.0.
BICR: blinded independent central review; HR: hazard ratio; PD-L1: programmed death-ligand 1; PFS: progression-
free survival.
Rao commented that these findings suggest that carboplatin–paclitaxel plus retifanlimab should be considered as a new global standard of care for first-line advanced SCAC. The regimen provides durable benefit, high disease control, and acceptable tolerability in a patient population that historically had limited effective treatment options.
Future Outlook: Immunotherapy and Beyond
Looking ahead, the therapeutic landscape for anal cancer continues to expand rapidly, with a number of trials exploring multiple immunotherapy strategies across disease stages.43-45 Rao commented that the ongoing trials of vaccines in combination with immunotherapy look particularly interesting, but suggested that more work is needed to investigate a possible role for targeted treatments in this disease area.
Present and Future Targets in Colorectal Cancer: Focus on KRAS and TGF-β
In the final presentation, Rao turned the attention of the audience towards CRC, which remains one of the most prevalent malignancies worldwide.46 Although advances in screening, surgery, chemotherapy, and targeted therapies have improved outcomes, metastatic disease continues to carry a poor prognosis.47 Five-year survival falls sharply once distant metastases are present, emphasising the need for more precise molecularly targeted approaches.48
The therapeutic landscape of CRC has become increasingly complex, with multiple signalling pathways under investigation. The approval of bevacizumab and cetuximab in 2004 signalled the arrival of targeted therapies in CRC,47 and since then, targeted therapies have transformed the treatment paradigm in CRC.47,49 As a result, multiple novel targeted therapies, directed against a diverse array of cellular pathways in CRC, are being explored in CRC.47,50 Among these, KRAS mutations represent a particularly important challenge and opportunity for innovation.
Within the KRAS-mutant population, the most common alterations include G12D, G12V, and G13D, while G12C accounts for only a small proportion.51 These mutations are associated with inferior prognosis and reduced sensitivity to anti-EGFR therapy.51 Despite decades of effort, direct targeting of RAS proteins was long considered impossible; however, Rao explained that the development of allele-specific inhibitors and degraders has changed this paradigm.
Several KRAS inhibition strategies are now being explored.52,53 Mutation-specific inhibitors selectively bind either the active, GTP-bound (‘on’) or inactive, GDP-bound (‘off’) conformations of KRAS, locking the protein in its inactive form.53,54 RMC-6236 is an oral RASMULTI(ON) tri-complex inhibitor that binds to cyclophilin A, resulting in a binary complex that potently binds to RAS(ON) to form a tri-complex, blocking downstream signalling.55 In another approach, proteolysis-targeting chimaeras direct the degradation of RAS proteins through the ubiquitin–proteasome system.56
The KRASG12D-selective degrader ASP3082 is currently under evaluation in a Phase I, open-label, multicentre study in adults with KRASG12D-mutant advanced solid tumours (NCT05382559).57,58 Meanwhile, the selective G12D inhibitor, INCB161734, has shown encouraging early-phase results, with manageable safety profiles and preliminary evidence of antitumour activity in patients with solid tumours.59 Collectively, these agents represent the first generation of rationally designed RAS-targeted therapies and are expected to reshape management strategies once mature efficacy data emerge.
Beyond KRAS, attention has turned to the TGF-β pathway, which plays a pivotal role in epithelial–mesenchymal transition and tumour progression.60 Overexpression of TGF-β is a key pathway in the development of CRC, promoting epithelial–mesenchymal transition, angiogenesis, and immunosuppression.61,62 Due to its role in tumour progression, TGF-β expression has been studied as a potential prognostic biomarker in CRC.61,63
Several drugs targeting TGF-β signalling are in development in CRC, although numerous drugs targeting TGF-β signalling have stopped development due to toxicity concerns/pleiotropic effects.62-66 Examples of promising candidates in this area include the bifunctional fusion protein targeting TGF-β and PD-L1, bintrafusp alfa, which demonstrated modest antitumour activity and a manageable safety profile in patients with heavily pretreated, advanced CRC.67 In addition, the first-in-class bispecific antibody, INCA33890, targets PD-1-positive cells only, thereby sparing normal tissues and reducing off-target effects.68-70 In a Phase I, open-label, multicentre study in participants with advanced or metastatic solid tumours, INCA33890 demonstrated encouraging activity, including responses in patients with liver metastases.71 Toxicities were largely immune-related and manageable, with fatigue, rash, pruritus, and diarrhoea as the most frequent adverse events. Importantly, severe treatment discontinuations remain uncommon, suggesting a favourable therapeutic window.71
Rao concluded her presentation, commenting that CRC remains a biologically diverse disease with substantial unmet need. The rapid expansion of KRAS inhibitors and tumour-specific TGF-β modulators represents one of the most dynamic areas of current oncology research. Together, these innovations signal a shift toward increasingly personalised, tumour-specific targeted strategies capable of addressing molecular subtypes that have long resisted conventional therapy.







