BACKGROUND AND AIMS
Gram stain (GS) and BioFire® FilmArray® Pneumonia Panel (PN; Biomérieux, Marcy-l’Étoile, France) are rapid methods of detecting bacterial pneumonia pathogens. PN is a highly sensitive and specific tool that rapidly identifies numerous organisms and is validated for use on all types of respiratory specimens.1 While not widely used for this purpose, recent studies have suggested that GS may be used to guide antibiotic de-escalation for pneumonia in which Pseudomonas aeruginosa (PsA) and methicillin-resistant Staphylococcus aureus (MRSA) are suspected.2 This study seeks to evaluate the sensitivity of GS versus PN and GS versus bronchoalveolar lavage (BAL) culture (cx) to determine the utility of GS for antibiotic de-escalation.
MATERIALS AND METHODS
This is a single center retrospective study from 1/2021–1/2024 on adults ≥18 years of age admitted for pneumonia. Respiratory sample GS sensitivity for detecting MRSA and PsA was measured using PN or BAL cx on the same specimen as reference standards. PN results were pooled for all respiratory specimen types (e.g., expectorated sputum, tracheal aspirate, and BAL) due to a small number of BAL specimens with PN done on them. Wilson 95% CIs were calculated for each measured test characteristic.
RESULTS
A total of 57 cases in the BAL cx group (mean age: 57 years) and 149 cases in the PN group (mean age: 58 years) were reviewed. In the BAL cx group, there were 11 cases of PsA, four cases of MRSA, and eight of any type of S. aureus as identified in the BAL cx. Meanwhile, PN identified 32 cases of PsA, 22 cases of MRSA, and 47 cases of any type of S. aureus.
The sensitivity of GS with BAL cx as the reference standard was 36% for PsA (95% CI: 15–65%); Figure 1A). The sensitivity for MRSA was comparable at 40% (95% CI: 12– 77%), with a general S. aureus sensitivity of 44% (95% CI: 19–73%). The sensitivity of GS using PN as the reference was 78% for PsA (95% CI: 61–89%), 82% for MRSA (95% CI: 61–93%), and 60% for S. aureus overall (95% CI: 45–72%; Figure 1B). Subgroup analysis of the PN specimens that were tracheal aspirates or from BAL showed similar sensitivities to the pooled PN results (Figure 1C). In the subgroup, GS sensitivity was 85% for PsA (n=13; 95% CI: 58–96%), 92% for MRSA (n=12; 95% CI: 65–99%), and 58% for S. aureus overall (n=24; 95% CI: 39–76%).
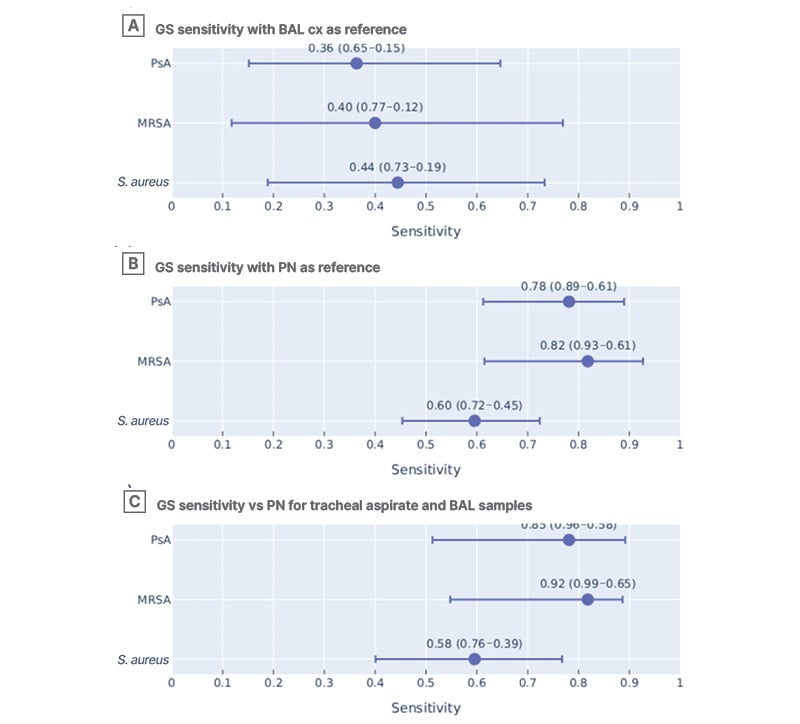
Figure 1: Gram stain sensitivity across organisms using different reference standards.
GS sensitivity using A) BAL cx and B) PN as the reference standards. C) GS sensitivity using PN as the reference only for the subgroup of samples that were tracheal aspirate or also had BAL cx done.
BAL: bronchoalveolar lavage; cx: culture; GS: Gram stain; MRSA: methicillin-resistant Staphylococcus aureus; PN: BioFire® FilmArray® Pneumonia Panel (Biomérieux, Marcy-l’Étoile, France); PsA: Pseudomonas aeruginosa; vs: versus.
Performance of PN was compared to formal culture from any source. For PsA (n=19), sensitivity was 100% (95% CI: 83–100%) with a specificity of 94% (95% CI: 74–99%). For MRSA (n=8), sensitivity was 100% (95% CI: 68–100%) with a specificity of 96% (95% CI: 62–99%).
CONCLUSION
GS had poor sensitivity for PsA, MRSA, and S. aureus in general compared to BAL cx. GS appeared to perform better compared to PN than to BAL cx, a difference likely due to false positives from normal respiratory flora found in the non-BAL specimens. However, even with the higher sensitivity in the PN group, GS would still miss at least 20% of true PsA and MRSA infections, which raises concerns about use in critically ill patients.







