Abstract
Plexiform neurofibromas (PNF) are benign peripheral nerve sheath tumours classically associated with neurofibromatosis Type 1 (NF1). Isolated PNFs in patients without clinical or genetic evidence of NF1 are exceptionally rare and may pose diagnostic and therapeutic challenges. This case report describes a female who first presented in childhood with a congenital solitary PNF of the left hemiface and, as an adult, demonstrated rapid regrowth following multiple excisions. Comprehensive genomic profiling identified KRAS p.K117N and an AKT1 in-frame indel (W80_T81>CRQRTSS) with no germline or somatic NF1 alteration, suggesting alternative oncogenic activation of rat sarcoma (RAS)–MAPK and PI3K–protein kinase B (AKT) pathways. Multimodal management included numerous surgeries and targeted therapy (trametinib plus pazopanib), which achieved partial reduction but was limited by toxicity and access constraints. Subsequent chemoradiotherapy conferred minimal additional benefit. The patient was referred to the Undiagnosed Diseases Network (UDN) of the National Institutes of Health (NIH) for further evaluation. This report highlights the importance of precise clinicopathological characterisation and broad molecular testing in atypical PNFs, and underscores gaps in consensus guidance for solitary, non-NF1 PNFs where surgery is not feasible. Precision oncology may offer rational options, although durable control remains challenging in highly aggressive and infiltrative lesions.
Key Points
1. Solitary plexiform neurofibromas (PNF) without clinical or genetic neurofibromatosis Type 1 are rare and challenging. This case showed rapid regrowth after multiple excisions and required multidisciplinary assessment beyond standard surgery. It is, to the authors’ knowledge, the first reported case from Mexico.2. Broad sequencing revealed KRAS p.K117N and an AKT1 in-frame indel, supporting alternative activation of rat sarcoma (RAS)–MAPK and PI3K–protein kinase B (AKT) pathways in non-neurofibromatosis Type 1 PNF.
3. Targeted therapy produced partial regression but was constrained by toxicity and access. Chemoradiotherapy offered limited additional benefit. The case underscores the lack of specific guidance for solitary PNF and the need for personalised decisions when complete resection is not feasible.
INTRODUCTION
Plexiform neurofibromas (PNF) are benign, often infiltrative tumours of the peripheral nerve sheath classically linked to neurofibromatosis Type 1 (NF1), appearing in about 30% of patients with this disease.1 However, a small subset, known as ‘isolated’ or ‘solitary’ PNFs, occurs in patients who do not meet the clinical diagnostic criteria or harbour the germline mutations associated with NF1.2
To date, the best epidemiologic study of solitary PNFs is the systematic review performed by Ho et al.,3 which identified 35 studies comprising 39 subjects with a total of 41 documented isolated mucocutaneous PNF cases.3
These present with a similar histopathologic architecture as NF1-associated PNFs but tend to be solitary, well-circumscribed, and benign, so diagnosis rests on clinical and genetic exclusion of NF1 and careful radiological–pathological correlation.4 Because their molecular and biological profiles differ from NF1-related PNFs, direct treatment extrapolation is uncertain. Surgery remains the mainstay, although local recurrence may occur.5
This report presents, to the authors’ knowledge, the first reported case in Mexico of a 17-year-old female with a recurrent, congenital, hemifacial, solitary PNF that harboured KRAS p.K117N and AKT1 in-frame indel (W80_T81>CRQRTSS), detailing its diagnostic work-up and multidimensional management.
CASE
A 17-year-old Mexican female presented to the authors’ centre with a congenital left hemifacial lesion and malformed left auricle previously treated in both Mexico and the USA. Family history for neurocutaneous disorders was negative. Personal history included multiple craniofacial procedures performed in childhood (auricular reconstructions 15–18 years prior to initial presentation; mastoidectomy with temporal lesion resection 13 years prior; excisions of anterior neck, tongue, and tonsillar masses 12 years prior; and removal of a temporal osteoma/chondroma 11 years prior). Nine years prior to initial presentation, a left cavernous internal carotid artery fusiform aneurysm was treated endovascularly after a successful balloon test occlusion, achieving complete occlusion with platinum coils and Onyx-34 (Medtronic, Dublin, Ireland), and no complications (Table 1).
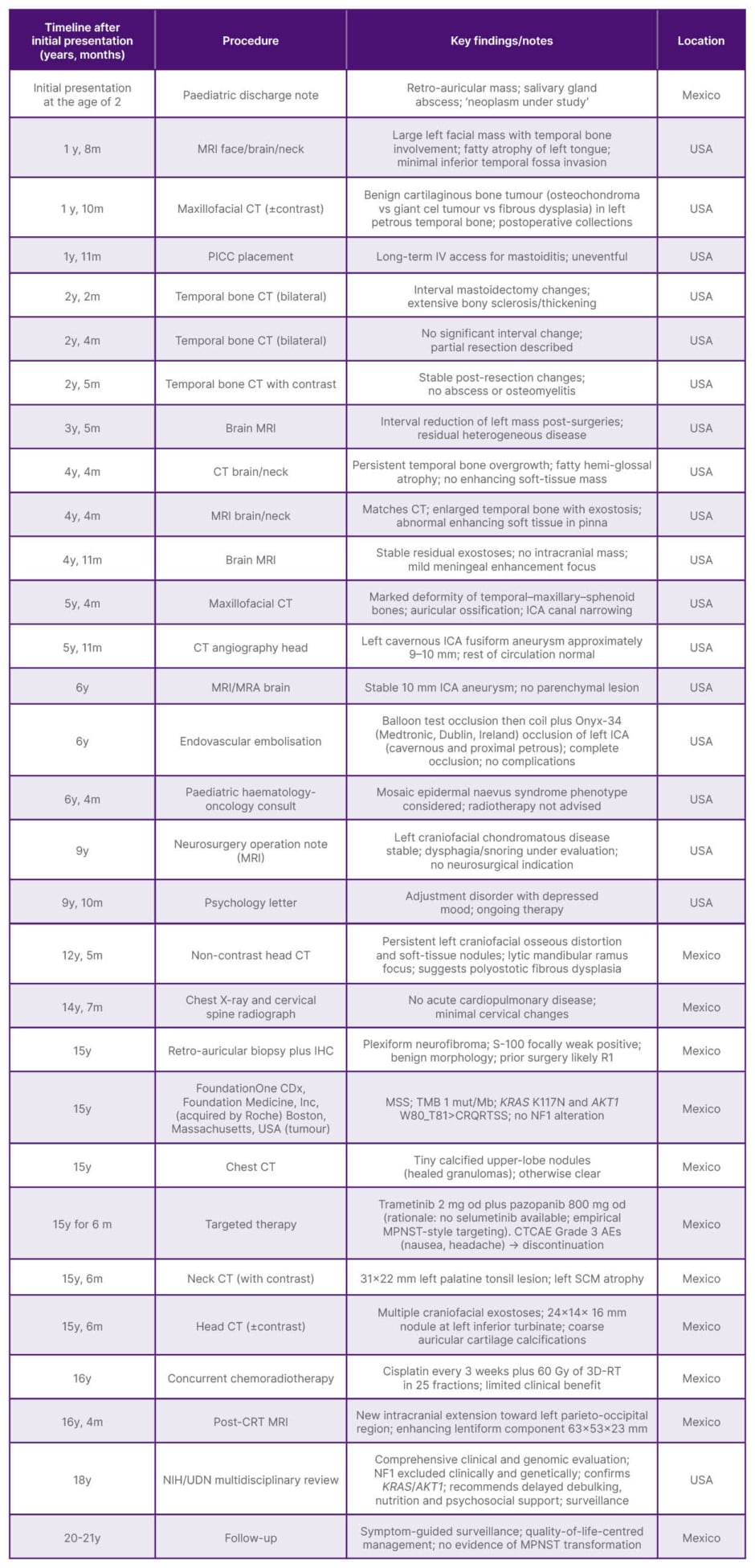
Table 1: Summary of patient’s procedures and findings.
AE: adverse event; CRT: chemoradiotherapy; CTCAE: Common Terminology Criteria for Adverse Events; ICA: internal carotid artery; IHC: immunohistochemistry; IV: intravenous; m: months; Mb: megabase; MPNST: malignant peripheral nerve sheath tumour; MRA: magnetic resonance angiography; MSS: microsatellite stable; mut: mutation; NF1: neurofibromatosis Type 1; NIH: National Institutes of Health; od: once daily; PICC: peripherally inserted central catheter; RT: radiotherapy; SCM: sternocleidomastoid muscle; TMB: tumour mutational burden; UDN: Undiagnosed Diseases Network; vs: versus; y: years.
Cross-sectional imaging from 2006–2014 consistently demonstrated a large, heterogeneously enhancing left facial mass with temporal bone overgrowth/exostoses extending into infratemporal, masticator, and parapharyngeal spaces; ossification of the left pinna; narrowing of the left carotid canal; and fatty atrophy of the posterior left tongue. Intracranial parenchyma remained unremarkable. Radiological differentials across reports favoured benign cartilaginous bone tumour/osteochondroma and polyostotic fibrous dysplasia; a paediatric haemato-oncology assessment in 9 years prior to initial presentation considered epidermal naevus syndrome (mosaicism).
After a period without specialist follow-up, a head CT without contrast performed 3 years prior in Mexico showed extensive left craniofacial osseous remodelling with soft-tissue nodules, including a dominant periorbital–malar component measuring approximately 61×60 mm, a lytic left mandibular ramus focus (24×18 mm), and hyperdense material along the left internal carotid artery, compatible with prior embolisation.
Retroauricular biopsy 1 month prior to initial presentation confirmed plexiform neurofibroma, showing plexiform proliferations of wavy spindle cells in a collagenous stroma without atypia or mitoses, and weak, focal S-100 immunoreactivity. The patient was thus referred to the authors’ centre.
After clinical assessment, tumour next-generation sequencing was indicated (1 month after initial presentation), demonstrating microsatellite stability, tumour mutational burden (one mutation per megabase), and co-occurring KRAS p.K117N and AKT1 W80_T81>CRQRTSS alterations, with no NF1 variant detected. A chest CT in the same month showed only tiny calcified upper-lobe nodules consistent with prior granulomatous disease. Head and neck CT 3 months later again documented left temporal bone enlargement with exostoses, a 31×22 mm left palatine tonsillar lesion, and a 24×14×16 mm nodule at the left inferior turbinate; long-standing fatty hemiglossal atrophy persisted.
Because the lesion was unresectable owing to vascularity and skull-base infiltration, an empirical, biology-based regimen was employed, given the aggressive behaviour, which initiated 1 month after initial presentation with trametinib 2 mg once daily plus pazopanib 800 mg once daily (selumetinib was unavailable locally). After approximately 6 months, imaging documented a partial radiological response (index component reduced from approximately 61×60 mm to approximately 31×22 mm) with symptomatic improvement. However, therapy was discontinued because of Common Terminology Criteria for Adverse Events (CTCAE) Grade 3 adverse events (notably nausea and headache) and financial constraints.
Alternative treatment with concurrent chemoradiotherapy (for 6 months the following year) was initiated, consisting of cisplatin every 3 weeks and external-beam radiotherapy to a total dose of 60 Gy in 25 fractions using 3D conformal radiation therapy. However, it yielded limited benefit, as post-treatment MRI nearly 1.5 years after initial assessment at the authors’ centre demonstrated intracranial extension towards the left parieto-occipital region with an enhancing lentiform component measuring 63×53×23 mm, together with persistent soft-tissue and osseous disease (Figure 1).
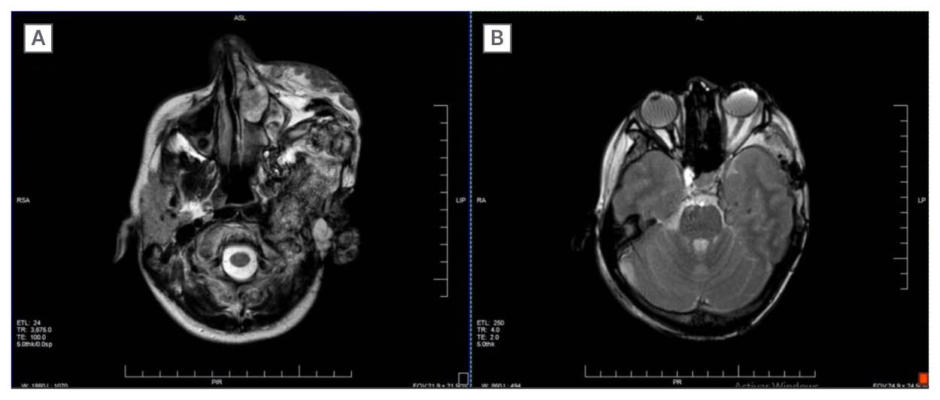
Figure 1: Axial MRI demonstrating extensive left hemifacial plexiform neurofibroma.
A) Heterogeneous, infiltrative soft-tissue and osseous involvement of temporal, maxillary, and zygomatic regions.
B) T2-weighted image showing mass effect with posterior displacement of the left orbital contents.
Because of the atypical phenotype (solitary PNF without clinical or genomic NF1) and suboptimal local control, a referral was made to the Undiagnosed Diseases Network (UDN) of the National Institutes of Health (NIH). Multidisciplinary NIH reassessment 3 years after the first assessment at the authors’ centre confirmed the prior KRAS and AKT1 somatic alterations without additional drivers, excluding NF1 syndrome clinically and genomically again. This concluded that, despite the extension and infiltrative behaviour of the lesion, resection would be the most appropriate disease-modifying option. Given malnutrition, anxiety, and functional impairment at that time, active surveillance with symptom-directed care was recommended as they continued to assess resection options. At the latest review, the clinical picture comprises persistent left hemifacial deformity with auricular ossification, reduced oral aperture, dysphagia, left facial palsy, ocular surface exposure symptoms due to incomplete eyelid closure, and reduced ipsilateral hearing. There is no clinical or histological evidence of malignant peripheral nerve sheath tumour transformation. Management focuses on surveillance, nutritional optimisation, ocular protection, and psychological support, with re-evaluation of surgical options as functional status allows.
DISCUSSION
Solitary PNFs are exceedingly rare entities that challenge the dogma that the ‘plexiform’ growth pattern is exclusively associated with NF1.6 Their clinical features, genetic landscape, pathogenesis, and therapeutic management remain unclear.7
Mean age of presentation was 19.6 years, with nearly 64% of the reported cases being paediatric patients and 49% within the first decade of life, and there was a slight male predominance (53.8% versus 46.2%) in the systematic review by Ho et al.3 The most common site was head and neck, followed by the trunk, hands and, less commonly, the lower limbs in cutaneous lesions, while 90% of mucosal lesions occurred in the oral cavity.3 In this case, the lesion originated at the left ear and was resected multiple times, but with limited efficacy, as the infiltrative behaviour of the tumour allowed regrowth multiple times.
The diagnosis of a solitary PNF is complex, as clinicians usually relate this entity to von Recklinghausen’s disease.8 Unfortunately, pathological assessment of the lesions resected during childhood was not available; however, it was probable that PNF was excluded from the differential diagnosis due to its lack of clinical criteria, as little was known about solitary PNFs at that time.9 However, after 2 decades, it has been recognised as a different disease with unique molecular and biological behaviour.10
Unlike PNFs in the NF1 setting, where biallelic NF1 loss and a characteristic low mutational burden dominate the molecular signature,1 the genomic landscape in isolated cases remains incompletely defined. Previous cases have discussed a possible NF1 mosaicism or segmental forms of NF1 that are clinically unapparent outside of the tumour tissue,11 demonstrating NF1 inactivation through an insertion of chromosomal bands (1p36-35 at 17q11.2) in one allele and a deletion in the other, leading to an isolated plexiform neurofibroma in a 13-year-old boy.12 However, more recent cases have proposed alternative NF1-independent pathways, like the case presented by Stallworth et al.,13 in which an activating KRAS mutation and an inactivating mutation in PHF6 were observed.
The detection in this tumour of an activating KRAS variant (p.K117N) together with an AKT1 in-frame indel provides a plausible mechanism for sustained pathway activation that phenocopies the biological consequences of NF1 loss.14 Although there is no approved, direct targeted therapy for KRAS p.K117N in this disease context, such alterations offer a mechanistic rationale for pathway-directed interventions (Figure 2).
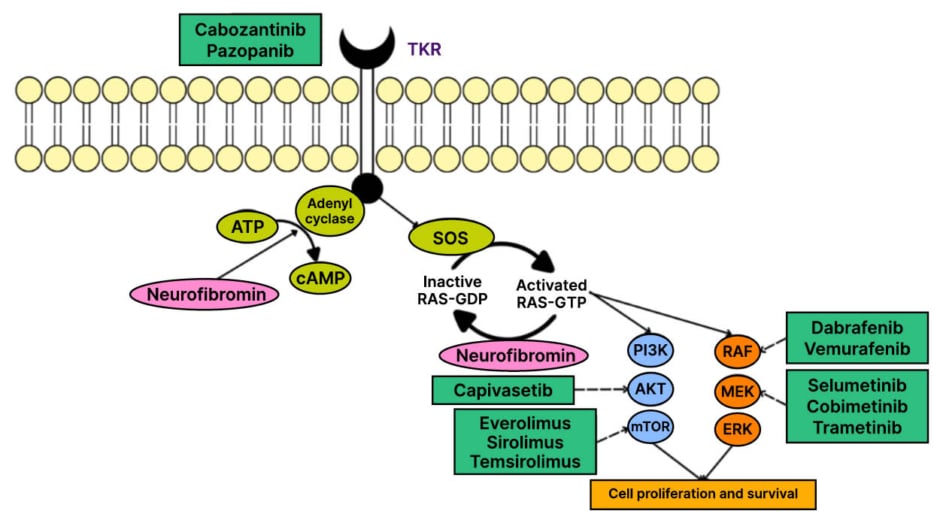
Figure 2: Rat sarcoma–MAPK and PI3K–protein kinase B–mTOR signalling with candidate therapeutic targets.
Dysregulation of RAS–MAPK and PI3K–AKT–mTOR signalling can drive proliferation; candidate drug targets are shown.
AKT: protein kinase B; cAMP: cyclic adenosine monophosphate; ERK: extracellular signal-regulated kinase; GDP: guanosine diphosphate; GTP: guanosine triphosphate; MEK: mitogen-activated protein kinase kinase; RAS: rat sarcoma; RAF: rapidly accelerated fibrosarcoma serine/threonine-protein kinase; SOS: son of sevenless protein; TKR: tyrosine kinase receptor.
Specifically, mitogen-activated protein kinase kinase (MEK) inhibition can dampen MAPK signalling downstream of KRAS, while anti-angiogenic blockade may modulate the hypervascular, stroma-dependent microenvironment that frequently characterises large, infiltrative PNFs.
In this context, over the past years, clinical activity of MEK inhibitors in NF1-associated PNFs has been demonstrated, with meaningful reductions in tumour volume and symptom burden (particularly in children), whereas earlier attempts with imatinib, cabozantinib, miR farnesyl-transferase inhibitors, or mTOR inhibition achieved only modest disease stabilisation.15 Extrapolating from this biology, trametinib was used to counteract KRAS-driven MAPK activation as selumetinib was unavailable in the authors’ country. Pazopanib, which targets vascular endothelial growth factor receptor/platelet-derived growth factor receptor/fibroblast growth factor receptor, was combined, given the pronounced vascularity and the expectation that reducing angiogenic signalling and stromal support could enhance disease control, which has been demonstrated in soft-tissue sarcomas.16 The combination achieved a partial radiological response consistent with pathway plausibility; however, durability was limited by toxicity and access constraints, underscoring real-world barriers even when a coherent biological strategy is available.
Given these considerations and the lesion’s unresectability, an alternative treatment with cisplatin-based chemoradiotherapy was considered despite not being recommended for NF1-related PNFs due to the risk of radiation-induced malignancy in susceptible tissues.15 However, as this was an unresectable isolated PNF with substantial symptoms, it was considered the best option after a multidisciplinary review and explicit consent from the patient, attending to organ-at-risk constraints and malignancy risk.17
Given the limited benefit this regimen provided, and without any options left in the authors’ country, referral to the NIH UDN was considered; however, the COVID-19 pandemic delayed the patient’s travel until 3 years after her initial presentation. After a comprehensive review, it was determined that it was indeed a solitary PNF, for which surgery remained the most promising option for durable local control. However, active surveillance was favoured given the current performance status and competing risks.18
This report has several limitations. Pathology material from childhood procedures performed abroad was unavailable, precluding central histopathological review and comparison across time. Access to MEK inhibitors formally approved for NF1-PNF (e.g., selumetinib, mirdametinib)19 was constrained in this setting, which influenced therapeutic choices. Although trametinib combined with pazopanib achieved a partial response, the experience reflects off-label use and should be interpreted cautiously; in similar cases, MEK inhibitors or multikinase inhibitors (e.g., cabozantinib) may be considered according to the tumour’s mutational profile, access, and risk–benefit assessment. However, from a patient-centred perspective, and acknowledging the scarcity of consensus documents specific to isolated PNF, it was considered that supportive care was not ancillary but central, providing structured nutritional optimisation, pain management, functional rehabilitation (including speech and swallowing), and embedded psychological support in the care plan until resection is feasible.
CONCLUSION
This case demonstrates that the diagnosis of solitary PNFs is a challenge, as it requires not only clinical assessment but also genomic testing. Moreover, it expands the clinical and genomic spectrum of isolated PNFs by documenting concomitant KRAS p.K117N and an AKT1 in-frame indel in a congenital, hemifacial lesion with aggressive regrowth and is, to the authors’ knowledge, the first solitary PNF reported from Mexico. It highlights how comprehensive molecular profiling can uncover non-NF1 drivers that justify pathway-directed therapy, while also revealing the current limitations in durability and access. In the absence of dedicated guidelines, management should be personalised, multidisciplinary, and explicitly quality-of-life-centred, reserving systemic or locoregional treatments for unresectable, progressive, or highly symptomatic disease and revisiting surgical options as patient factors evolve.







