Abstract
Introduction: Pinealoblastomas are rare, aggressive, Grade 4 tumours of the pineal gland, predominantly affecting children. Their occurrence in adults is exceedingly rare, posing significant diagnostic and therapeutic challenges due to the lack of standardised management protocols.
Case Presentation: The authors present the case of a 23-year-old woman with a 3-month history of hearing loss. Brain MRI revealed a large (6.5×5 cm), unresectable pineal region tumour causing obstructive hydrocephalus. Biopsy confirmed the diagnosis of pinealoblastoma. Initial attempts at radiotherapy were precluded by severe agitation. A multidisciplinary team decision led to treatment with induction chemotherapy (cisplatin and etoposide) followed by craniospinal radiotherapy (54 Gy total dose) using the volumetric modulated arc therapy technique.
Outcomes: The patient tolerated the treatment well, with significant improvement in her neuropsychiatric status. A post-therapeutic MRI at 3 months showed an 80% tumour regression (near-complete remission) and resolution of hydrocephalus. The patient made a full neurological recovery and successfully resumed her university studies.
Conclusion: This case demonstrates that a sequential approach of induction chemotherapy followed by high-dose radiotherapy can be a highly effective strategy for managing unresectable pinealoblastoma in adults, leading to excellent oncological and functional outcomes. It underscores the need for adaptive, multidisciplinary management and highlights the potential of non-surgical modalities.
Key Points
1. Pinealoblastoma is a rare, aggressive, Grade 4 tumour of the pineal gland that is exceptionally uncommon in adults and poses significant diagnostic and therapeutic challenges due to a lack of standardised management protocols.2. The authors describe an adult case of unresectable pinealoblastoma managed successfully with induction chemotherapy followed by high-dose craniospinal radiotherapy, achieving near-complete remission and full neurological recovery.
3. A sequential strategy combining induction chemotherapy and craniospinal radiotherapy can be effective for unresectable adult pinealoblastoma, emphasising the importance of multidisciplinary, adaptive management when surgery is not feasible.
INTRODUCTION
The pineal gland is a small endocrine gland that regulates the circadian rhythm by secreting melatonin. Tumours of the pineal region represent less than 1% of all intracranial neoplasms, among which pinealoblastoma is distinguished as a primitive neuroectodermal tumour.1
Pinealoblastomas represent between 24–50% of pineal parenchymal tumours and occur mainly in infants and young children and occasionally in adults.1 Considered the most aggressive among pineal parenchymal tumours, they are classified as Grade 4 tumours according to the WHO classification.2 Due to compression of the cerebral aqueduct, pinealoblastoma is almost systematically associated with obstructive hydrocephalus. Furthermore, this tumour presents a high risk of leptomeningeal and extracranial dissemination.
Pinealoblastoma cases occurring in adults are extremely rare and represent less than 10% of cases reported in the literature.3 Due to this rarity, the data available to establish standardised management of the disease remain limited, and no consensus currently exists on the optimal combination of multimodal treatment involving surgery, radiotherapy, and/or chemotherapy.
OBJECTIVE
In this work, the authors describe the case of an unresectable giant pinealoblastoma in a 23-year-old patient. The treatment consisted of induction chemotherapy followed by craniospinal radiotherapy. The post-treatment outcome was favourable, with almost complete remission after the end of treatment.
This case illustrates a particularly rare presentation of pinealoblastoma in an adult patient, which is unusual given that the majority of cases occur in children. Furthermore, the tumour was deemed unresectable, and the favourable outcome following a sequential strategy of induction chemotherapy followed by craniospinal radiotherapy highlights an alternative, non-surgical approach with excellent results.
CASE PRESENTATION
A 23-year-old, right-handed female university student with no significant past medical history presented with a primary complaint of bilateral progressive hearing loss over 3 months. Neurological examination showed no cranial nerve deficits, papilledema, or focal motor signs. Baseline blood tests were within normal limits. There were no reported headaches, visual disturbances, nausea, or vomiting at initial presentation. A detailed timeline of the patient’s care is presented in Table 1.
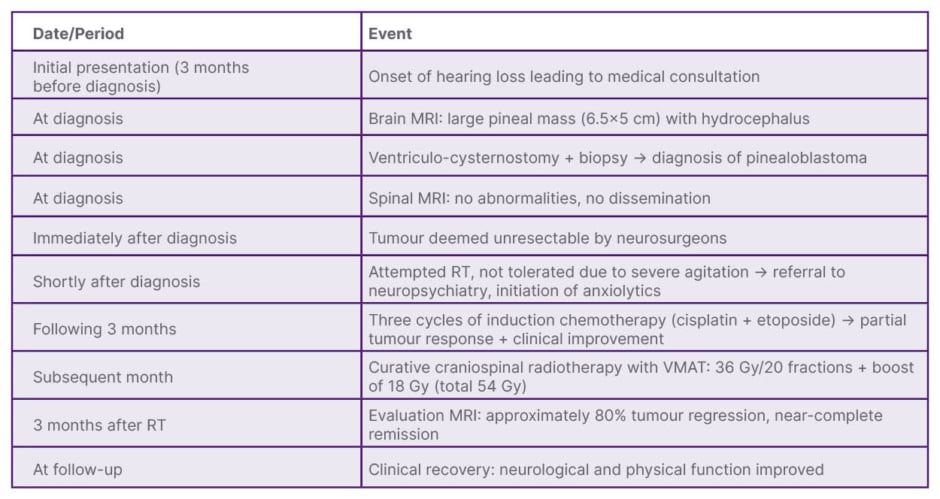
Table 1: Timeline of patient’s care.
RT: radiotherapy; VMAT: volumetric modulated arc therapy.
Brain MRI revealed a large (6.5×5 cm), heterogeneous, cystic mass in the pineal region with calcifications. The mass compressed the cerebral aqueduct, causing triventricular obstructive hydrocephalus (Figure 1). The patient underwent a ventriculocisternostomy to relieve hydrocephalus and a concurrent biopsy of the pineal lesion.
Figure 1: Tumour iconography on initial brain MRI.
A) T1: heterogeneous expansive process centred on the pineal gland with moderate enhancement after gadolinium injection.
B) T2: heterogeneous cystic tissue structure with hypo calcification signals.
C) T2: compression of the aqueduct of the brain responsible for triventricular hydrocephalus.
Histopathological examination showed a highly cellular tumour composed of sheets of small, round, monomorphic cells with hyperchromatic nuclei and scant cytoplasm, with evidence of rosette formation (Figure 2). Immunohistochemical analysis showed tumour cell positivity for synaptophysin and neuron-specific enolase, with negative glial fibrillary acidic protein staining. The Ki-67 proliferative index was estimated at 40–50%, consistent with the highly proliferative nature of pinealoblastoma. These findings helped distinguish pinealoblastoma from other pineal region tumours such as pineocytoma, germ cell tumours, and ependymoma. Although no molecular or cytogenetic analyses were performed in this case, such investigations are increasingly recognised as valuable for identifying prognostic and therapeutic biomarkers.
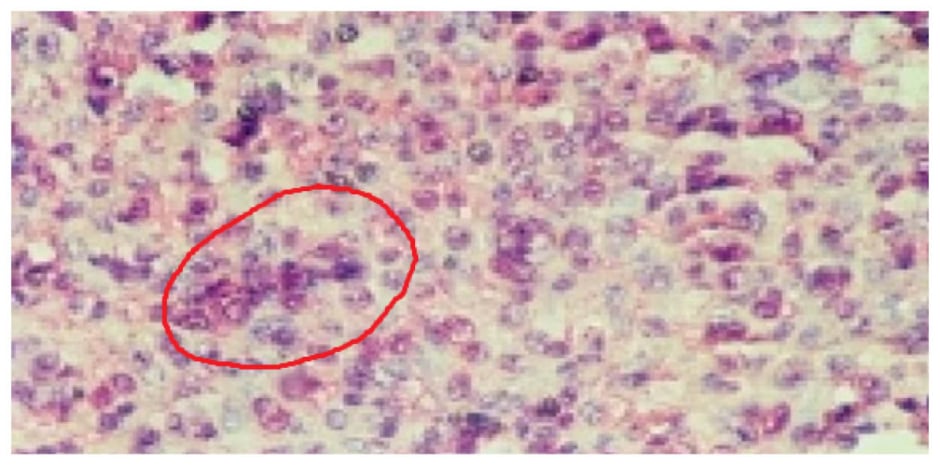
Figure 2: Microscopic image of pinealoblastoma made up of round monomorphic cells with reduced cytoplasm equipped with anisokaryotic and hyperchromatic nuclei, arranged in a sheet, clump, and rosette.
A spinal MRI showed no evidence of leptomeningeal dissemination. Given the tumour’s large size and proximity to the brainstem, it was deemed unresectable by neurosurgery. An initial attempt to administer radiotherapy failed due tosevere treatment-related agitation, requiring neuropsychiatric intervention and anxiolytics. A multidisciplinary tumour board decided on a novel sequence for this context:
Induction Chemotherapy (3 months following diagnosis): Three cycles of cisplatin and etoposide, resulting in partial tumour response.
Radiotherapy (1 month later): Following a good partial response and clinical improvement, the patient received craniospinal irradiation to 36 Gy in 20 fractions, with a simultaneous integrated boost to the tumour bed (18 Gy), to a total dose of 54 Gy. This was delivered using the volumetric modulated arc therapy technique (Figure 3).
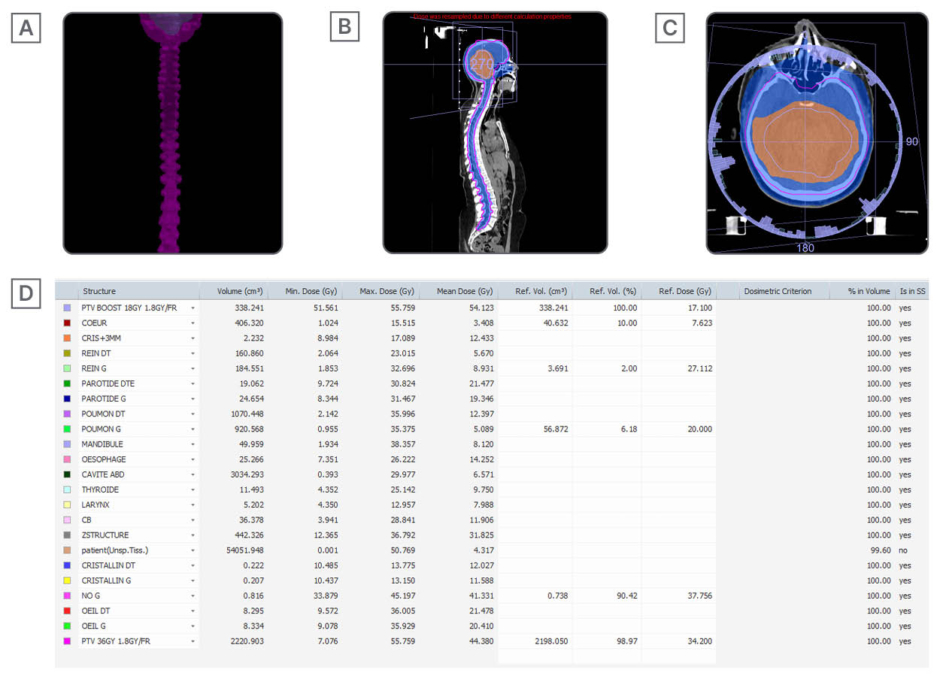
Figure 3: Curative craniospinal radiotherapy using volumetric modulated arc therapy technique at a total dose of 36 Gy with dose overprinting on the tumour at a dose of 18Gy in normal fractionation of 1.8GY/fr.
A), B), and C) Target craniospinal volumes + boost and 95% isodoses.
D) Statistics of doses delivered on target volumes and constraints at the level of organs at risk
The primary outcome was radiological response. A follow-up MRI 3 months post-radiotherapy showed approximately 80% regression of the tumour mass. The secondary outcome was clinical status; the patient’s hearing loss and agitation resolved completely. She recovered full neurological function and resumed her academic studies without limitation. No acute or long-term toxicities from treatment were reported at the 3-month follow-up.
DISCUSSION
Pinealoblastomas represent a major therapeutic challenge in neuro-oncology, where a lack of consensus on optimal management persists.4 The authors’ observation of an unresectable giant pinealoblastoma in a 23-year-old patient illustrates the adaptive strategies required in this rare pathology.
The initial tumour extent and neuropsychiatric status compromising radiotherapy guided the authors’ therapeutic sequence. This choice is consistent with the data of Barlas et al.,4 where a non-surgical approach (stereotactic biopsy, cerebrospinal fluid diversion, and radiotherapy) resulted in an overall survival of 80% at 28 months in six patients with unresectable tumours. While several studies confirm that complete surgical resection followed by radiotherapy remains the ideal option,3,5 the authors’ case highlights the effectiveness of alternatives when surgery is impossible.
The dose of radiotherapy delivered to the primary tumour has a significant impact on overall survival. The recommended total dose should exceed 50 Gy.6 Conventional fractionation is recommended, preferably using recent techniques such as intensity-modulated radiotherapy. Regarding target volume delineation, although the most adopted strategy is based on craniospinal irradiation followed by local boost, some authors have reported treatment focused only on the tumour site. However, a cranial dose of ≥40 Gy was associated with improved survival.7
The use of neoadjuvant chemotherapy has proven crucial. As Lombardi et al.8 pointed out, adult protocols remain extrapolated from paediatrics, with radio-chemotherapy combinations (alkylating agents/platinum/vincristine) offering 60–70% progression-free survival at 5 years for non-metastatic forms.8 The authors’ choice of cisplatin–etoposide is consistent with the observations of Biswas et al.,9 where this regimen was the most frequently used in adult pinealoblastoma, while the results of Lee et al.7 are a reminder that the impact of chemotherapy remains variable according to subpopulations.
This success should not mask the molecular heterogeneity of pinealoblastoma. Recent work has identified distinct subgroups, including tumours with MYC proto-oncogene amplification sensitive to mTOR inhibitors, and those with RB1 (RB transcriptional corepressor 1) loss vulnerable to WEE1 inhibitors. In this context, clinical trials evaluating targeted therapies (such as NCT02574728 and NCT02095132) or immunotherapies could potentiate conventional regimens. Systematic molecular characterisation becomes essential to explain exceptional responses and guide personalised strategies.10
In addition to pinealoblastomas, other tumours of the sellar and suprasellar regions, such as craniopharyngiomas,11 may also present with similar clinical features and therapeutic challenges. Recent advances in radiomics have further expanded diagnostic possibilities, providing imaging-based biomarkers that may complement histopathological evaluation in rare central nervous system tumours.12
CONCLUSION
This observation validates the efficacy of an induction chemotherapy sequence followed by high-dose craniospinal radiotherapy for unresectable pinealoblastoma. The cisplatin–etoposide regimen merits evaluation in prospective trials dedicated to adults, combined with molecular analysis to identify candidates for innovative therapies. The integration of these targeted approaches represents the future of treatment for these rare tumours.
PATIENT PERSPECTIVE
Being diagnosed with a pinealoblastoma was a difficult experience, especially since surgery was not an option. Thanks to chemotherapy followed by radiotherapy, I experienced significant tumour regression and neurological recovery. This treatment allowed me to resume my studies and regain hope for the future.







