Abstract
Allergic asthma is the most common phenotype of asthma and presents with various clinical subtypes and clusters, emphasising the importance of personalised treatments in its management. The disease has an IgE-mediated inflammatory course that may be triggered by many agents, such as pollens and nonsteroidal anti-inflammatory drugs. The allergic asthma patients are relatively young, with early-onset asthma and frequent exacerbations. The primary goal of this literature review is to provide a deeper insight into different patient groups and allergic asthma phenotypes, as well as to discuss treatment options accordingly. Triggering factors and clinical presentation of patient groups are also covered in this study.
INTRODUCTION
Asthma is a common disease in both children and adults, and it is estimated that there are >300 million people with asthma worldwide.1 Due to the heterogenous origin of the disease, various asthma phenotypes have been identified which can be broadly classified as allergic and non-allergic. The World Asthma Phenotypes (WASP) study started in 2016 and includes five centres, one from each of the UK, New Zealand, Brazil, Ecuador, and Uganda. The findings of this study will be released soon and will help the scientific community to understand the characterisation and the distribution of asthma phenotypes in different geographic areas.2 However, allergic asthma is still known to be the most common phenotype of asthma and its prevalence is increasing, while the prevalence of non-allergic asthma remains stable.3
Th2-related, IgE-mediated eosinophilic airway inflammation due to allergen exposure is the classical pathway of allergic asthma.1 Atopy is common in patients with allergic asthma and the prevalence of both allergic rhinoconjunctivitis and atopic dermatitis are also high.4 It is also seen more commonly in the young male population.4 There are several atopic clusters identified under atopic asthma and several triggers, such as pollens and aspirin, which are related to distinguished forms of allergic asthma.4 Asthma treatment is recommended to be made in accordance with the phenotypic characteristics of each patient. Therefore, these differences in allergic asthma phenotypes are important for proper management of the disease. The analysis of the phenotypes suggest personalised novel therapeutic strategies, such as omalizumab for allergic asthma management. These new treatments are shown to be beneficial in slowing down the allergic inflammation. However, there is no definitive treatment to halt the allergic inflammation in asthma. This review aims to provide analyses of the literature on allergic asthma phenotypes, mainly focussing on the adult population. The effect of biological therapies will also be discussed.
CLINICAL ALLERGIC ASTHMA PHENOTYPES
In phenotyping studies, clustering of asthma phenotypes requires certain variables related to clinics, such as demographics, natural history, and airway inflammation. A recent study using data from ADEPT and U-BIOPRED cohorts identified four phenotypes.5 Among these four groups, the Phenotype 2 was a ‘moderate, hyper-responsive, eosinophilic’ phenotype with moderate asthma control, mild airflow obstruction, and Th2 predominant airway inflammation. This group was highly atopic and mostly consisted of moderate persistent asthmatics with high hyperreactivity.5 Cluster analysis of the COREA cohort identified four asthma phenotypes: Clusters 1, 2, 3, and 4, which are smoker’s asthma, severe obstructive asthma, early-onset atopic asthma, and late-onset mild asthma, respectively.6 Atopy rates in these clusters were 34.0% in Cluster 1, approximately half of the patients in Cluster 2, and about two thirds of the patients in Cluster 3. Mean forced expiratory volume in 1 second (FEV1) was the lowest in Cluster 2 and mean age at onset was lowest in Cluster 3.6 Haldar et al.7 performed k-means cluster analysis in a mild-to-moderate asthma population managed in primary care and a severe asthma population managed in secondary care. Cluster analysis of both populations described a subgroup with early-onset atopic asthma. The subgroup was associated with a significantly greater number of previous hospital attendances, asthma exacerbations requiring oral corticosteroids, and eosinophilic airway obstruction. Siroux et al.8 identified four asthma phenotypes from cluster analysis of two large European population-based epidemiological studies. Phenotypes defined as ‘active treated allergic childhood-onset asthma’ and ‘inactive/mild untreated allergic asthma’ were characterised with atopy, airway hyperreactivity, and active disease at the time of examination. Boudier et al.9 investigated asthma phenotypes and the transitions across these phenotypes via cluster analysis of three big European 10-year follow-up cohorts. Two atopic phenotypes were identified in the study: the first cluster was composed of asthma patients with allergic sensitisation and no or few respiratory symptoms, while the second cluster was composed of allergic, highly symptomatic asthma patients with high hyperreactivity. The probabilities of being in the same phenotype group at each time point varied from 54% to 88% across phenotypes. During the 10-year period, transitions between phenotypes occurred rarely. Results of all these studies show that allergic asthma patients are clinically young, have early-onset mild-to-moderate asthma with high bronchial hyperreactivity, airway eosinophilic inflammation, and frequent asthma exacerbations.
Recent studies have suggested that allergic asthma prevalence in patients aged >55 years might be more common than reported.10 The study by Ozturk et al.11 showed that the prevalence of atopy was 21% in an elderly population with asthma; these patients were most commonly sensitised to Dermatophagoides pteronyssinus (44.4%). In the study by Park et al.,12 four clusters were identified in elderly patients enrolled from the COREA cohort. Atopy rate was higher in the elderly cohort compared to the primary younger cohort (31.8% versus 18.9%). Atopy rate among clusters was between 15.6% and 26.4%. The highest atopy rate was detected in Cluster 3, which included patients with high smoking rates and reduced lung function. Sano et al.13 classified elderly asthmatic patients into three clusters: asthma-predominant, asthma-obstructive airway disease overlap, and asthma–emphysema overlap. The overall group atopy rate was 46% and the asthma–emphysema overlap group had the most frequent atopic status. Ozyigit Pur et al.14 found that older patients with allergic asthma had worse asthma control and lower values for FEV1 compared to younger ones. In the study by Lombardi et al.,15 sensitisation to at least one allergen was observed in 52.4% of elderly asthma patients and their results were similar to those of Ozturk et al.,11 who found that house dust mites were the most common allergens in elderly patients with asthma.
Phenotypic characteristics of severe asthma are usually defined as low lung function and high exacerbation rate with no atopy.16 However, recent studies defined allergic phenotype in severely asthmatic patients.17,18 In the study of Shaw et al.,17 the European U-BIOPRED adult severe asthma cohort was classified into four groups; atopy rates were 78.3% in non-smokers with severe asthma, 71.3% in smokers and ex-smokers with severe asthma, 92.3% in non-smokers with mild-to-moderate asthma, and 46.2% in healthy controls who were non-smokers. Patients with severe asthma had more symptoms and exacerbations with lower lung function and higher eosinophil count, despite the treatment with higher doses of inhaled or oral corticosteroids, compared to patients with mild or moderate disease. In the study by Wu et al.,18 six subject clusters were identified; four of these clusters had skin test reactivity to multiple allergens. Severe asthma patients were in Clusters 3 and 6. Patients in Cluster 3 had frequent symptoms, normal FEV1 values, little inflammation, and a high degree of allergic sensitisation. Patients in Cluster 6 had early-onset asthma and were more symptomatic with the lowest lung function, frequent asthma attacks, and more sinusitis incidence. The cross-sectional analyses of the Belgian severe asthma registry showed that most severe asthmatics are female and atopic.19 Eosinophilic asthma was the predominant phenotype and the most common comorbidities were rhinitis and chronic rhinosinusitis in the severe asthma group.
Borders of asthma phenotypes are drawn by clinical findings such as age at disease onset, severity of disease, and presence of comorbid conditions. However, present cluster studies are using the presence of atopy as the sole discriminating criterion of allergic asthma causing overlaps between allergic and non-allergic phenotypes. To elucidate the association between allergic phenotype and symptoms, severity, comorbidities, and response to therapy, allergic sensitisation characteristics, and allergenic triggers of each patient should be determined. To discuss the issue further, trigger-induced asthma phenotypes should be examined and, in the following section, specific trigger-induced asthma phenotypes, such as aspirin-exacerbated respiratory disease (AERD) and pollen asthma, will be discussed.
TRIGGER-INDUCED ASTHMA PHENOTYPES
Pollen Asthma
Pollen allergy has been found in 80–90% of childhood asthmatics and 40–50% of adult-onset asthmatics.20 Thunderstorm asthma epidemics that are triggered by grass pollen rupture in the atmosphere have been mentioned in the literature.20 Pollen exposure causes an increase in airway inflammation during pollen season, especially in central airways that do not affect the degree of bronchial obstruction.21 However, pollen exposure also affects small airways. Patients who were mono-sensitised to weed pollen were found to have different degrees of airway wall thickness and small airway obstruction.22 Airway wall thickness is negatively correlated with airway obstruction and positively correlated with the duration of rhinitis.22 It has been reported that asthma-related emergency department visits have been increasing due to high atmospheric pollen and mould levels during spring.23 Although early asthmatic responses were similar, the study of Boulet et al.24 showed that late asthmatic responses induced by house dust mites were stronger than those induced by pollens.
Allergic clinical symptoms and their severity may vary according to the type of allergen. Findings of the PERFILAR I and II studies indicated that clinical characteristics of asthma could be statistically different depending on the allergen type to which the patient is sensitised.25 Seasonal allergens are found to be associated with a longer duration until the development of asthma. Parietaria is more important than Olea and Gramineae as a risk factor for developing nonspecific bronchial hyperresponsiveness.26 In a recent study, cough was detected as a predominant symptom in adults with pollen-induced asthma.27 Celikel et al.28 analysed the records of 922 patients diagnosed with seasonal allergic rhinitis retrospectively to determine the risk factors for asthma in adults. In the study, the patients with seasonal allergic rhinitis were divided into four groups: no sensitisation, mono-pollen sensitisation, poly-pollen sensitisation, and mite sensitisation. When compared with the poly-pollen sensitisation group, no sensitisation and mite sensitisation groups had a higher risk of asthma, whereas the mono-pollen sensitisation group was unlikely to have any other allergic disease including asthma.
Aspirin-Exacerbated Respiratory Disease
The prevalence of AERD in adult-onset asthma patients is 7.4%.29 Its prevalence is twice as high in patients with severe asthma.29 Aspirin intolerance can be detected in 20% of patients with adult-onset asthma without any sinus disease.29,30 In this phenotype, patients have both nasal polyps and sinusitis with an allergic reaction involving upper and lower respiratory tracts after ingestion of aspirin or any other nonsteroidal anti-inflammatory drug (NSAID) that inhibits cyclooxygenase-1.30 Differing from the typical characteristics of allergic asthma phenotype, AERD is more common in females, rarely clusters in families, and develops in the third decade of life. However, two thirds of the patients with AERD have a history of atopy.30 In a recent study, the atopy rate among the patients with AERD was detected to be 84%, which was higher than the atopy rate in patients with chronic rhinosinusitis with nasal polyps (CRSwNP) and CRSwNP-asthma overlapping patients.31 It was also reported that patients with both CRSwNP and asthma had significantly more severe sinonasal inflammation and were significantly more likely to have oral corticosteroid-dependent severe asthma.
Some AERD subclasses have been reported in the literature and this heterogeneity is similar to that of allergic asthma patients. Bochenek et al.32 identified four classes within the AERD phenotype: Class 1 is asthma with a moderate course, intensive upper airway symptoms and blood eosinophilia; Class 2 is asthma with a mild course, relatively well controlled, and with low health care use; Class 3 is asthma with a severe course, poorly controlled, and with severe exacerbations and airway obstruction; and Class 4 is poorly controlled asthma with frequent and severe exacerbations in female subjects. In this study, atopy rate was 52.2%. However, atopic status did not affect distribution of patients among classes. Karakaya et al.33 defined two phenotypes in aspirin intolerant patients. Asthma patients are found to be associated more with the presence of a nasal polyp and/or rhinosinusitis, smoking history, and food allergy or food intolerance. In another study of the same group, asthma in patients with NSAID hypersensitivity was associated with female sex, sinonasal polyposis/polyp surgery, rhinitis or rhinosinusitis, NSAID-induced rhinitis, asthma or a blended reaction pattern, immediate reaction following NSAID intake, self-reported history of food allergy, and family history of asthma.34 However, atopy was not associated with asthma in patients with NSAID hypersensitivity. Clinical characteristics of different asthma subgroups are summarised in Figure 1.
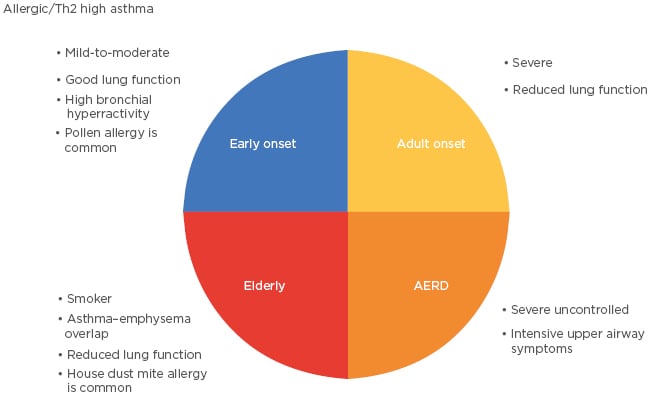
Figure 1: Clinical characteristics of different asthma subgroups.
AERD: aspirin-exacerbated respiratory disease; Th2: T-helper 2 cell.
BIOLOGICAL THERAPIES IN ALLERGIC ASTHMA
Since allergic asthma is a Th2-related, IgE-mediated disease, therapies targeting type 2 cytokines may have an effect on allergic asthma patients. Following consistent positive efficacy results of clinical trials on biological treatments, therapies targeting IgE, IL-5, IL-13, IL-4 receptor A, and thymic stromal lymphopoietin (TSLP) are either approved now or will be approved soon for allergic asthma.35 Revised Global Initiative for Asthma (GINA) 2019 guidelines recommend an add-on anti-IgE treatment for severe allergic asthma and anti-IL5, anti-IL-5R, or anti-IL-4R for severe eosinophilic asthma.1 In the following section, biologicals, including omalizumab, mepolizumab, reslizumab, benralizumab, and dupilumab, will be discussed in terms of their effects on different allergic asthma phenotypes.
OMALIZUMAB
Omalizumab is an anti-IgE monoclonal antibody that was approved by the US Food and Drug Administration (FDA) in 2003 and by the European Medicines Agency (EMA) in 2005 as an add-on treatment for patients aged >12 years of age with severe persistent allergic asthma.36 It is the first biological treatment for allergic asthma that selectively binds to circulating IgE and suppresses asthma symptoms by preventing the binding of IgE to effector cells.36
Since 2003, consistent evidence on the effectiveness of omalizumab has been presented and now it is in use for patients aged >6 years old who have severe uncontrolled asthma. Its efficiency rate in severe allergic asthma is approximately 61%.37 Within this phenotype, multi-sensitised allergic asthma patients with high baseline eosinophil levels before the treatment seem to respond better to omalizumab therapy.38 Allergic asthma patients who have high FeNO level, sputum, and nasal mucosa eosinophilia may also be positive responders to omalizumab.39 However, STELLAIR study found the response rate to be similar in both subgroups of severe allergic asthma patients with high (≥300 cells/μL) and low (<300 cells/μL) eosinophil counts.40 A recent study suggests that the patients with higher baseline serum IgE levels, shorter disease duration, and higher blood eosinophils may have a delayed benefit from omalizumab therapy and current non-responders can be evaluated again after between 16–32 weeks of treatment.41
Huang et al.42 showed that the beneficial effect of omalizumab was high in severe allergic asthma patients who have IL-33, IL-25, and TSLP aggravated type 2-high endotype. Responsive patients were mostly females and non-smokers who had higher eosinophilic airway inflammation and FeNO levels, worse asthma control, and lower forced vital capacity when compared with the non-responsive ones. Clinical efficacy of omalizumab is also high in severe persistent allergic asthma patients aged ≥50 years.43 A post hoc exploratory analysis of the PROSPERO study44 demonstrated that the patients with asthma–chronic obstructive pulmonary disease overlap (ACO) and a smoking history had similar improvements in asthma control when compared to patients without ACO when treated with omalizumab over 48 weeks. The optimal duration of omalizumab treatment is unclear. Findings of a recent study show that therapeutic effects of omalizumab use for 6 years may persist after discontinuation of therapy in 60% of patients for at least 4 years.45 However, after the reintroduction of omalizumab, some patients may not respond to treatment and 20% of the previous responders may fail to respond to the reintroduction of omalizumab.46
Mepolizumab
Mepolizumab is a fully humanised monoclonal IgG1 antibody that binds IL-5 and prevents it from binding its receptor. It was approved by the FDA and EMA in 2015 as maintenance treatment for severe eosinophilic asthma in patients aged ≥12 years.1 In 2007, the first large-scale, multicentre, double-blind, placebo-controlled trial using 250 or 750 mg intravenous (IV) mepolizumab at monthly intervals failed to demonstrate any positive clinical endpoint including morning peak expiratory flow, FEV1, daily β2-agonist use, symptom scores, exacerbation rates, and quality of life measures in moderate persistent asthma.47 In this study, 85% of the patients had allergic asthma that was confirmed by skin prick test positivity to animal dander, mite, or pollen. Subsequent studies including Dose Ranging Efficacy and Safety with Mepolizumab (DREAM)48 and Mepolizumab as Adjunctive Therapy in Patients with Severe Asthma (MENSA)49 investigated the efficacy of mepolizumab in eosinophilic asthma patients and determined exacerbation rate to be the primary outcome. With the change of inclusion criteria and primary endpoint, DREAM study demonstrated the positive effect of mepolizumab on the frequency of asthma exacerbations in patients with severe, exacerbation-prone, and eosinophilic asthma with a blood eosinophil count >300 cells/μL. Cluster analysis of the DREAM study showed that the reduction in exacerbations was significantly greater in patients who received mepolizumab with raised eosinophils and low airway reversibility.50 The patients in these clusters were more atopic and had more comorbid sinusitis and nasal polyposis.
The MENSA study confirmed the positive efficacy of mepolizumab on the reduction of asthma exacerbation rates in severe eosinophilic asthma. Relevant reductions in exacerbation frequency in patients with a blood eosinophil count >150 cells/μL were also noted.51 A significant glucocorticoid-sparing effect of mepolizumab in severe eosinophilic asthma was shown by the Steroid Reduction with Mepolizumab Study (SIRIUS).52 Post hoc analyses of the MENSA and SIRIUS studies were performed to evaluate the effect of mepolizumab in patients with severe eosinophilic asthma previously treated with omalizumab.53 In MENSA, mepolizumab reduced the rate of exacerbations by 57% (prior omalizumab) and 47% (no prior omalizumab) versus placebo. In the SIRIUS, reductions in oral corticosteroid use was found to be significant and independent of prior omalizumab use. These post hoc analyses indicate that patients with severe allergic asthma with elevated blood eosinophilia respond positively to mepolizumab regardless of prior use of omalizumab.
Reslizumab
Reslizumab is a humanised anti-IL-5 IgG4 monoclonal antibody that was approved by the FDA and the EMA in 2016 as add-on maintenance treatment for severe asthma patients with an eosinophilic phenotype and aged ≥18 years.54 The first large Phase IIb study55 of reslizumab evaluated the clinical efficacy of reslizumab at 3 mg/kg and 4 weekly IV doses in patients who had refractory eosinophilic asthma. Enrolled patients had confirmed airway reactivity, induced eosinophil sputum counts of >3%, and were on a high-dose inhaled corticosteroid and a second controller. Patients receiving reslizumab showed significant reductions in sputum eosinophils, improvements in airway function, and a trend towards better asthma control than those receiving placebo. Improved asthma control was significant in patients with nasal polyps. However, comorbid aspirin sensitivity rate was very low (6%) among this subgroup.
Analysis of two key Phase III multicentre studies reported a significant, larger reduction in asthma exacerbations in late onset (≥40 years) asthma patients than early-onset disease after 52-week reslizumab therapy.56 Compared to early-onset asthma patients, late-onset asthma patients were less atopic and had more nasal polyps. A single-blind, placebo-controlled sequential trial investigated the treatment response of weight-adjusted IV reslizumab in patients previously treated with 100 mg subcutaneous mepolizumab monthly for at least 1 year. Ten prednisone-dependent patients with asthma (five males; mean age: 50.9±7.6 years; mean BMI: 28.9±4.9) who presented with elevated blood and sputum eosinophilia (sputum eosinophils >3% and blood eosinophils >300 cells/μL) were included in the study. Interestingly, the authors found that the weight-adjusted IV reslizumab was superior to the fixed-dose subcutaneous mepolizumab in controlling asthma. Although the interpretation of this data is limited, study results suggest that biologicals targeting IL-5 may have different effects in different subgroups of severe eosinophilic asthma, such as prednisone-dependent asthma.
Benralizumab
Benralizumab is a humanised IgG1 monoclonal antibody that binds to the IL-5 receptors, leading to the apoptosis of basophils and eosinophils. Following three Phase III trials, SIROCCO, CALIMA, and ZONDA, benralizumab was approved in the USA and Europe in 2017 for patients aged ≥12 years with severe asthma who have an eosinophilic phenotype.57-59 The SIROCCO57 and CALIMA58 trials assessed the effect of benralizumab on asthma exacerbations over 48 and 56 weeks, respectively. Subjects were randomised to receive 30 mg of subcutaneous benralizumab every 4 weeks, or every 4 weeks for the first 3 doses then every 8 weeks, or placebo. When compared to placebo, both benralizumab dosages significantly lowered exacerbation rates and improved FEV1 in patients with a baseline blood eosinophil count of at least 300 cells/μL. Efficacy and safety of benralizumab for eosinophilic asthma patients with low blood eosinophil counts (≥150 cells/μL) were also shown in a sub-analysis of SIROCCO and CALIMA studies.60
The subgroups analysis of SIROCCO and CALIMA was according to age (<18 years, 18–65 years, and ≥65 years), sex, BMI (≤35 kg/m2 or >35 kg/m2), baseline oral corticosteroid use, number of exacerbations in the previous year (two, three, or four), race, nasal polyposis, atopic status, and smoking history.61 This showed that larger reductions in exacerbation rates and greater improvements in FEV1 were associated with a history of more frequent exacerbations. It is also found that larger FEV1 improvements were associated with oral corticosteroid use and history of nasal polyposis.61 However, decreases in exacerbation rates were found to be similar in high or low IgE and atopic or nonatopic groups.62 The third trial, ZONDA,59 investigated steroid sparing effect of benralizumab and showed that 28-week benralizumab use decreased median final oral glucocorticoid dose by 75% compared to baseline dose.
Mepolizumab, reslizumab, and benralizumab seem to have similar effects on symptom control and exacerbation rate reduction in patients with severe eosinophilic asthma when they are used in correct doses.63 However, GINA guidelines recommend assessing the response to biological therapy after 4 months and switching between different Th2 targeted therapies when little or no response is observed with a previous one.1
Dupilumab
Dupilumab is a human IgG4 antibody against the IL-4 receptor α-subunit, blocking the signalling of IL-4 and IL-13.64 In the USA, the FDA approved dupilumab for the treatment of moderate-to-severe atopic dermatitis in adults that is uncontrolled with topical medications in March 2017. Then, through the results of three randomised, double-blind, placebo-controlled trials with about 2,800 patients who were >12 years of age and presented with uncontrolled persistent asthma, dupilumab received further approval by the FDA. It was thereby allowed for use in an add-on maintenance therapy in patients with moderate-to-severe asthma aged ≥12 years with an eosinophilic phenotype or with an oral corticosteroid-dependent asthma in October 2018.64-67 The first of these trials65 enrolled 769 patients who were receiving treatment with medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist. It randomised them 1:1:1:1:1 to receive either 200 mg or 300 mg every 2 weeks or every 4 weeks, or placebo. Treatment with dupilumab for a period of 12 weeks and 24 weeks resulted in significant increases in FEV1 and decreases in severe asthma exacerbations compared to placebo. Percentage of patients with nasal polyposis were higher in the subgroup with eosinophil counts >300 cells/μL. However, dupilumab significantly improved lung function and reduced the rate of severe exacerbations independently from baseline eosinophil counts.
The second trial66 enrolled 1,902 patients who were ≥12 years of age with uncontrolled asthma. The patients were randomised 2:2:1:1 ratio to receive add-on subcutaneous dupilumab at a dose of 200 or 300 mg every 2 weeks or matched-volume placebos for 52 weeks. At Week 12, increased FEV1 levels and decreased severe asthma exacerbation rates were observed in the dupilumab receiving group. The mean age of the entire group was 47.9±15.3 years. Ongoing atopic or allergic condition rate was 80% and greater benefits were detected in patients with higher baseline levels of eosinophils.
In the third trial,67 210 patients with severe asthma were randomised to receive add-on treatment with dupilumab or placebo. The dosing was 300 mg or placebo every 2 weeks for 24 weeks. At Week 24, 48% of patients in dupilumab group had completely stopped using oral corticosteroids, compared to 25% of those in the placebo group. Dupilumab use also had positive effects on decreasing severe asthma exacerbations and improving lung function. Greater benefits were observed in patients with higher baseline blood eosinophil counts.
As supported by the increasing evidence found in different phenotypes of allergic asthma, new molecules and approaches other than anti-IgE, anti-IL-5, and anti-IL-4R will also shape the future of allergic asthma management, as seen with anti-TSLP, anti-IL-33, and anti-IL-25. However, which clusters in allergic asthma phenotypes are responders to these treatments is still an open question and further studies are needed.
Eligibility criteria, suggested initial dose, route and trial, and common side effects of biological treatments are summarised in Table 1 and different treatment options in different allergic asthma clusters are shown in Figure 2.
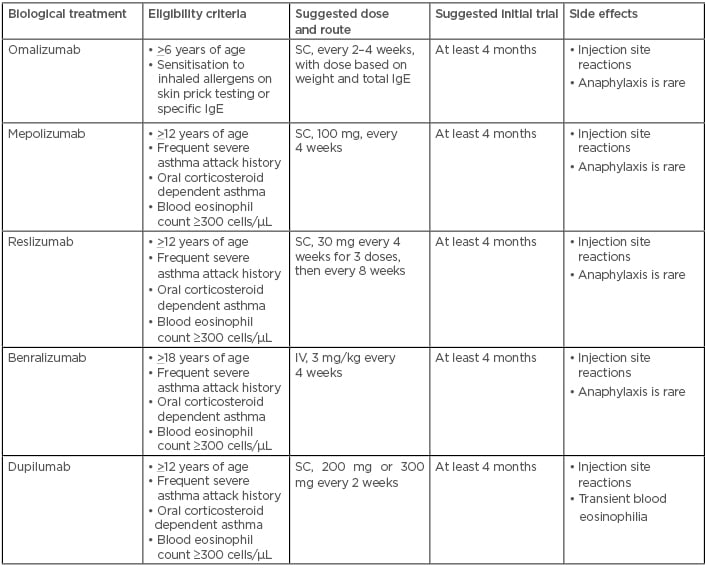
Table 1: Eligibility criteria, suggested initial dose, route and trial, and common side effects of biological treatments for allergic asthma.
Adapted from the GINA 2019 Difficult to Treat and Severe Asthma Pocket Guide.68 IgE: immunoglobulin E; IV: intravenous; SC: subcutaneous.
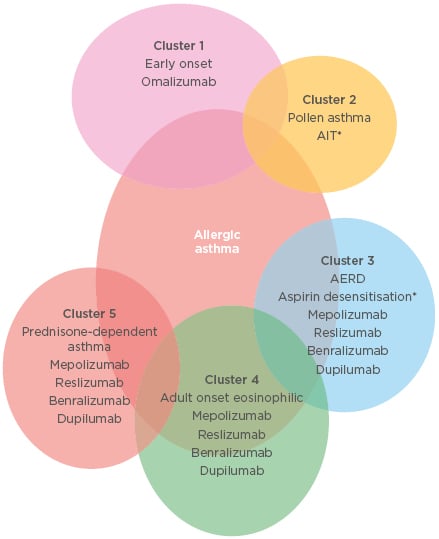
Figure 2: Different treatment options in different allergic asthma clusters.
*Forced expiratory volume in 1 second ratio >70% predicted. AERD: aspirin-exacerbated respiratory disease; AIT: allergen immunotherapy.
CONCLUSION
The data on allergic asthma is rapidly increasing. However, there are still missing pieces in the puzzle of allergic asthma. Research will help to find those pieces and to understand the clinical and biological differences in patients with allergic asthma. This will lead to the development of novel therapeutic drugs aiming to stop allergic airway inflammation. Some of the drugs that are currently in use, including omalizumab, have proven to be beneficial in the treatment of allergic asthma phenotype. Some of those drugs, such as mepolizumab, reslizumab, benralizumab, and dupilumab, need time to prove beneficial effects in different allergic asthma phenotypes.





